
(a)
Interpretation:
Chemical equation for the conversion of given
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
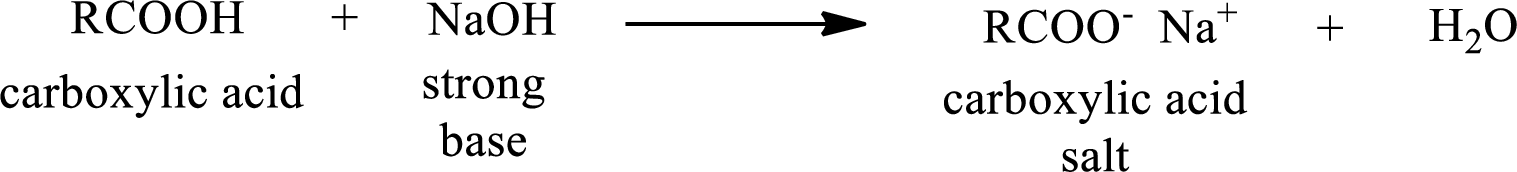
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
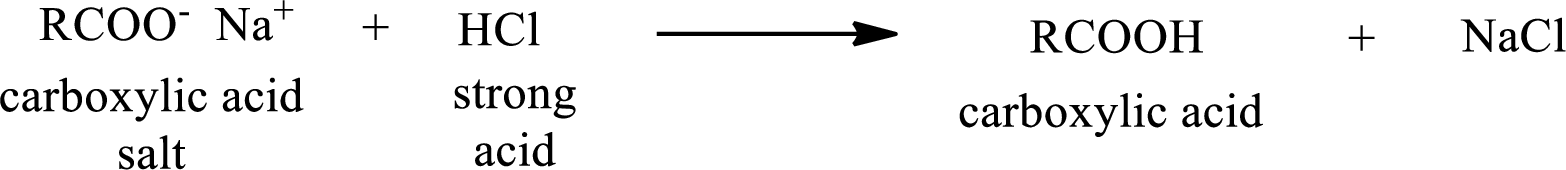
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(a)
Explanation of Solution
Given carboxylic acid salt is sodium butanoate. The structure of sodium butanoate can be given as shown below,
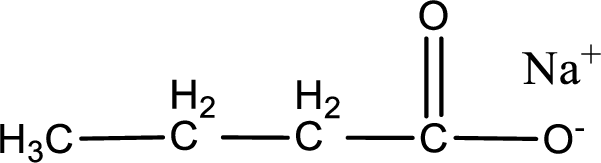
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
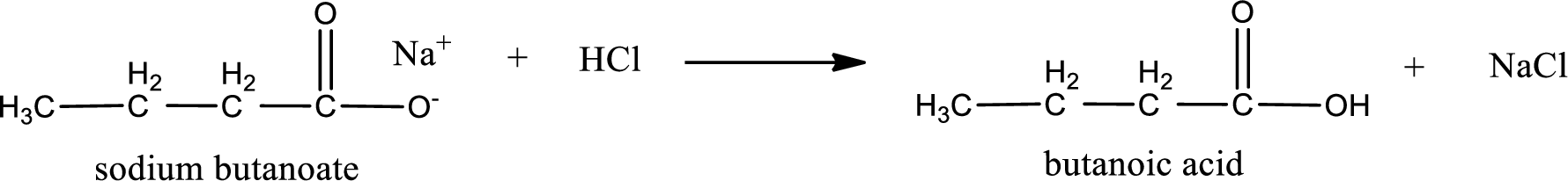
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(b)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
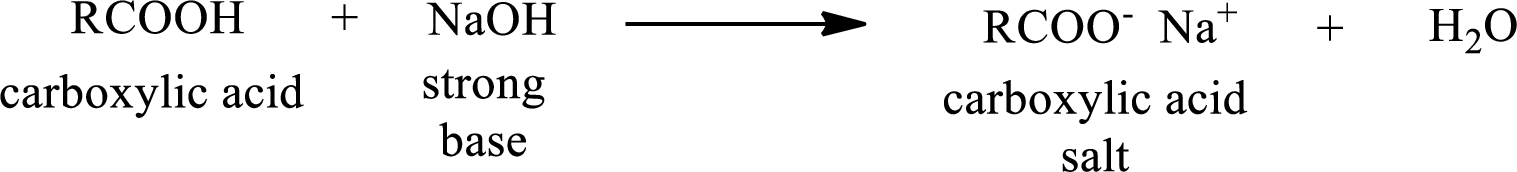
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
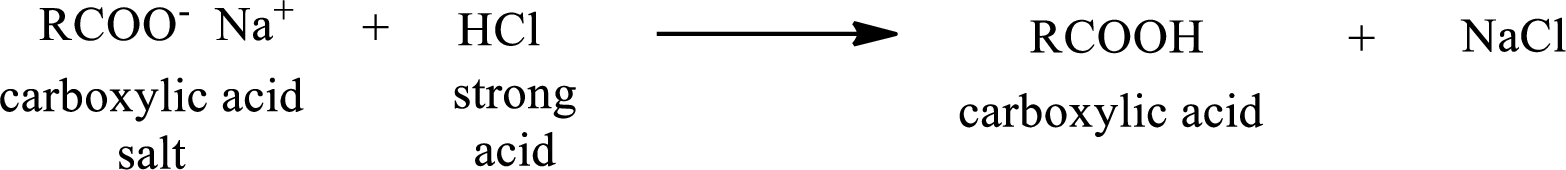
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(b)
Explanation of Solution
Given carboxylic acid salt is potassium oxalate. The structure of potassium oxalate can be given as shown below,
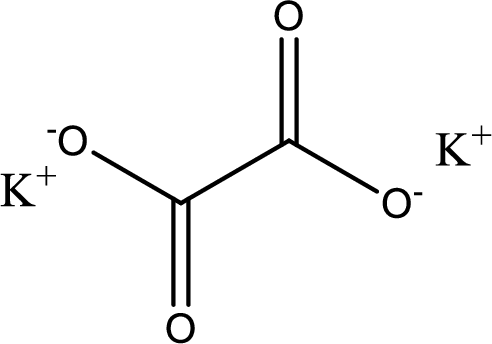
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
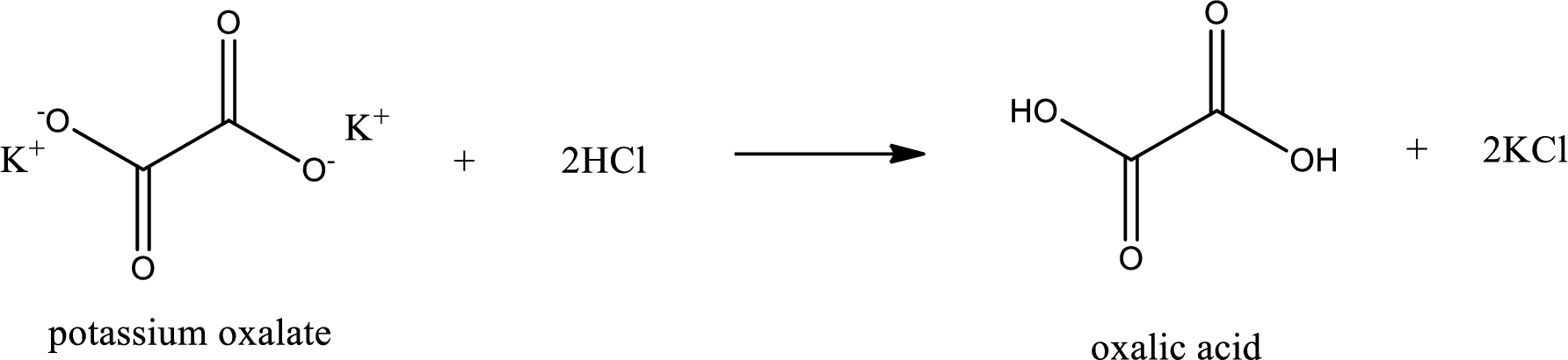
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(c)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
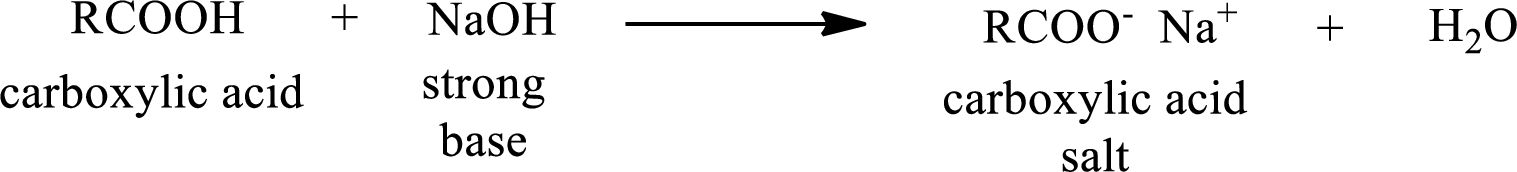
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
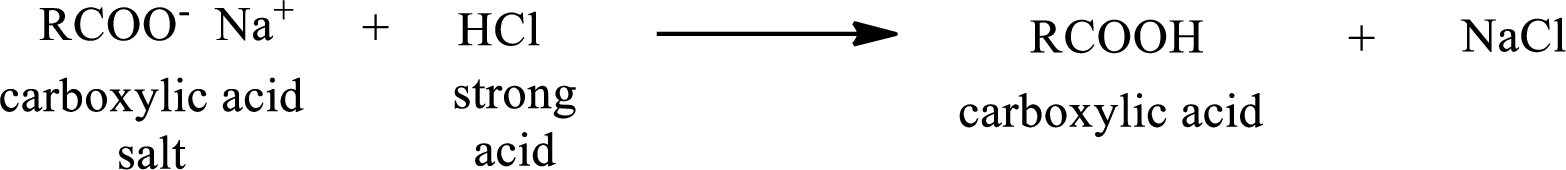
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(c)
Explanation of Solution
Given carboxylic acid salt is calcium malonate. The structure of calcium malonate can be given as shown below,
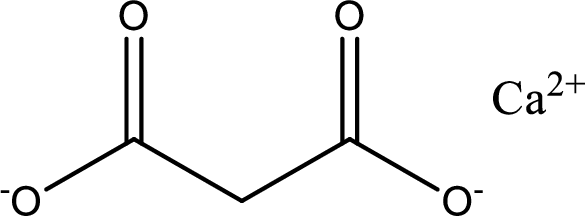
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
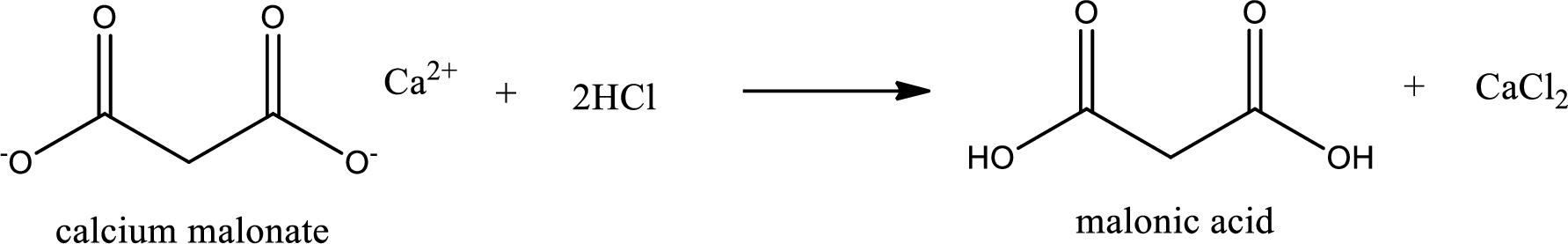
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(d)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
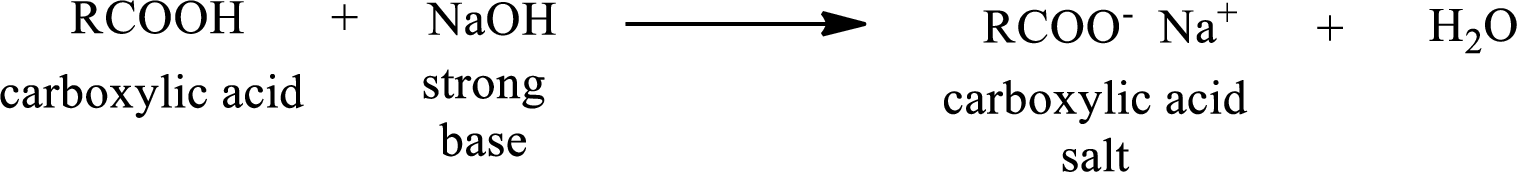
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
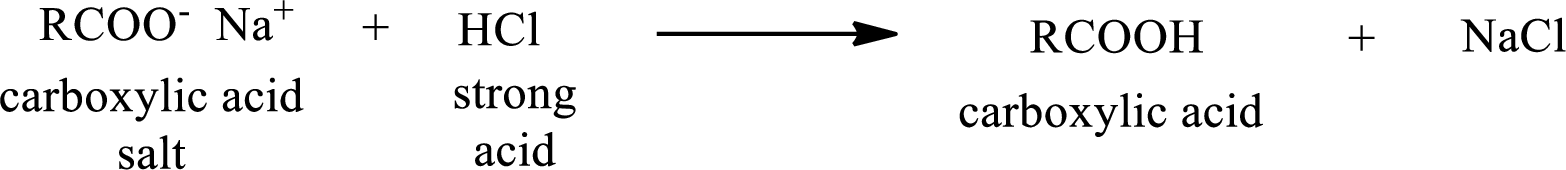
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(d)
Explanation of Solution
Given carboxylic acid salt is sodium benzoate. The structure of sodium benzoate can be given as shown below,
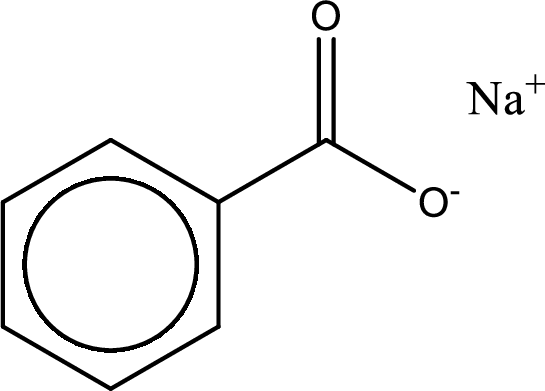
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
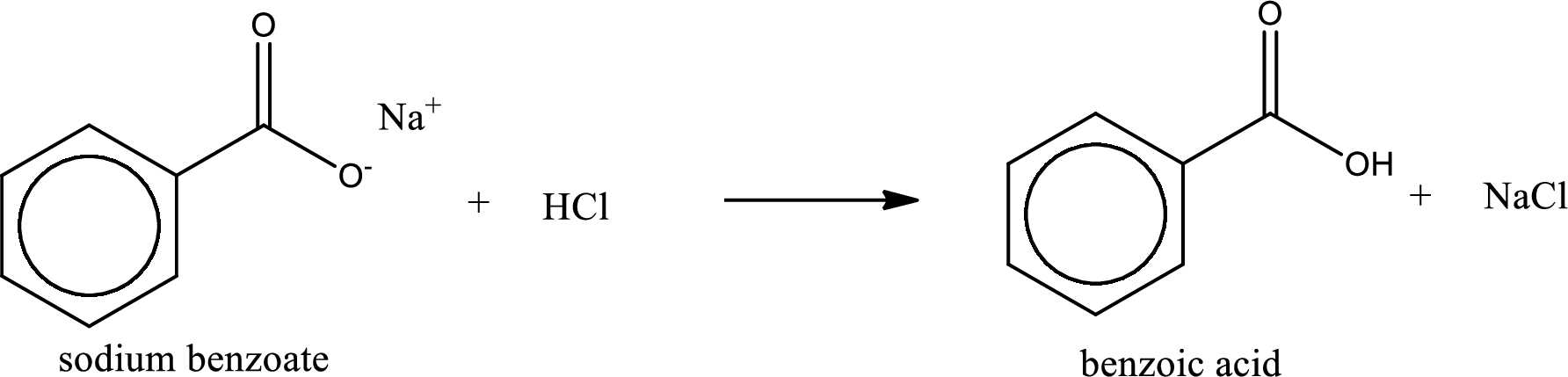
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- What is behavioral adaptarrow_forward22. Which of the following mutant proteins is expected to have a dominant negative effect when over- expressed in normal cells? a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function b. mutant Grb2 protein that cannot bind to RTK c. mutant RTK that lacks the extracellular domain d. mutant PDK that has the PH domain but lost the kinase function e. all of the abovearrow_forwardWhat is the label ?arrow_forward
- Can you described the image? Can you explain the question as well their answer and how to get to an answer to an problem like this?arrow_forwardglg 112 mid unit assignment Identifying melting processesarrow_forwardGive only the mode of inheritance consistent with all three pedigrees and only two reasons that support this, nothing more, (it shouldn't take too long)arrow_forward
- Oarrow_forwardDescribe the principle of homeostasis.arrow_forwardExplain how the hormones of the glands listed below travel around the body to target organs and tissues : Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forward
- What are the functions of the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardDescribe the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardPlease help me calculate drug dosage from the following information: Patient weight: 35 pounds, so 15.9 kilograms (got this by dividing 35 pounds by 2.2 kilograms) Drug dose: 0.05mg/kg Drug concentration: 2mg/mLarrow_forward
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage






