
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16.1, Problem 1QQ
Interpretation Introduction
Interpretation:
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
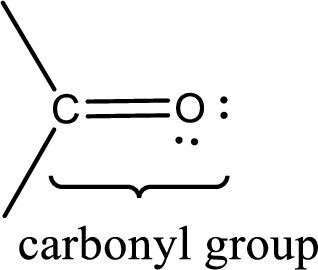
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
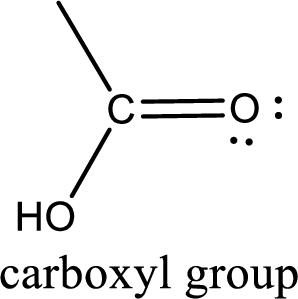
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
E
Organic Chemistry
Maxwell
Draw the correct products, in either order, for the ozonolysis reaction:
1) O3, CH2Cl2, -78 °C
Product 1
+
Product 2
2) Zn, HOAc
Draw product 1.
Select Draw Templates More
C
H
O
presented by M
Draw product 2.
Erase
Select Draw Templates M
/ # #
c
✓ edict the products of this organic reaction:
----
။
A
CH3–C−NH–CH2–C−CH3 + KOH
?
Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more
than one product, draw them in any arrangement you like, so long as they aren't touching.
If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area.
Explanation
Check
Click anywhere to draw the first
atom of your structure.
C
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibili
Predict the product of this organic reaction:
A
HO-C-CH3 + CH3NH2
P+ H2O
Specifically, in the drawing area below draw the condensed structure of P.
If there is no reasonable possibility for P, check the No answer box under the drawing area.
Explanation
Check
Click anywhere to draw the first
atom of your structure.
m
Chapter 16 Solutions
General, Organic, and Biological Chemistry
Ch. 16.1 - Prob. 1QQCh. 16.1 - Prob. 2QQCh. 16.1 - Prob. 3QQCh. 16.1 - Prob. 4QQCh. 16.1 - Prob. 5QQCh. 16.2 - Prob. 1QQCh. 16.2 - Prob. 2QQCh. 16.2 - Prob. 3QQCh. 16.2 - Prob. 4QQCh. 16.2 - Prob. 5QQ
Ch. 16.3 - Prob. 1QQCh. 16.3 - Prob. 2QQCh. 16.3 - Prob. 3QQCh. 16.4 - Prob. 1QQCh. 16.4 - Prob. 2QQCh. 16.4 - Prob. 3QQCh. 16.5 - Prob. 1QQCh. 16.5 - Prob. 2QQCh. 16.5 - Prob. 3QQCh. 16.6 - Prob. 1QQCh. 16.6 - Prob. 2QQCh. 16.7 - Prob. 1QQCh. 16.7 - Prob. 2QQCh. 16.7 - Prob. 3QQCh. 16.8 - Prob. 1QQCh. 16.8 - Prob. 2QQCh. 16.8 - Prob. 3QQCh. 16.8 - Prob. 4QQCh. 16.9 - Prob. 1QQCh. 16.9 - Prob. 2QQCh. 16.10 - Prob. 1QQCh. 16.10 - Prob. 2QQCh. 16.11 - Prob. 1QQCh. 16.11 - Prob. 2QQCh. 16.11 - Prob. 3QQCh. 16.12 - Prob. 1QQCh. 16.12 - Prob. 2QQCh. 16.12 - Prob. 3QQCh. 16.12 - Prob. 4QQCh. 16.13 - Prob. 1QQCh. 16.13 - Prob. 2QQCh. 16.14 - Prob. 1QQCh. 16.14 - Prob. 2QQCh. 16.14 - Prob. 3QQCh. 16.15 - Prob. 1QQCh. 16.15 - Prob. 2QQCh. 16.15 - Prob. 3QQCh. 16.16 - Prob. 1QQCh. 16.16 - Prob. 2QQCh. 16.16 - Prob. 3QQCh. 16.17 - Prob. 1QQCh. 16.17 - Prob. 2QQCh. 16.18 - Prob. 1QQCh. 16.18 - Prob. 2QQCh. 16.18 - Prob. 3QQCh. 16.19 - Prob. 1QQCh. 16.19 - Prob. 2QQCh. 16.19 - Prob. 3QQCh. 16.19 - Prob. 4QQCh. 16.20 - Prob. 1QQCh. 16.20 - Prob. 2QQCh. 16.20 - Prob. 3QQCh. 16.20 - Prob. 4QQCh. 16 - Prob. 16.1EPCh. 16 - Prob. 16.2EPCh. 16 - Prob. 16.3EPCh. 16 - Prob. 16.4EPCh. 16 - Prob. 16.5EPCh. 16 - Prob. 16.6EPCh. 16 - Prob. 16.7EPCh. 16 - Prob. 16.8EPCh. 16 - Prob. 16.9EPCh. 16 - Prob. 16.10EPCh. 16 - Prob. 16.11EPCh. 16 - Prob. 16.12EPCh. 16 - Prob. 16.13EPCh. 16 - Prob. 16.14EPCh. 16 - Prob. 16.15EPCh. 16 - Prob. 16.16EPCh. 16 - Prob. 16.17EPCh. 16 - Prob. 16.18EPCh. 16 - Prob. 16.19EPCh. 16 - Prob. 16.20EPCh. 16 - Prob. 16.21EPCh. 16 - Prob. 16.22EPCh. 16 - Prob. 16.23EPCh. 16 - Prob. 16.24EPCh. 16 - Prob. 16.25EPCh. 16 - Prob. 16.26EPCh. 16 - Prob. 16.27EPCh. 16 - Prob. 16.28EPCh. 16 - Prob. 16.29EPCh. 16 - Prob. 16.30EPCh. 16 - Prob. 16.31EPCh. 16 - Prob. 16.32EPCh. 16 - Prob. 16.33EPCh. 16 - Prob. 16.34EPCh. 16 - Prob. 16.35EPCh. 16 - Prob. 16.36EPCh. 16 - Prob. 16.37EPCh. 16 - Prob. 16.38EPCh. 16 - Prob. 16.39EPCh. 16 - Prob. 16.40EPCh. 16 - Determine the maximum number of hydrogen bonds...Ch. 16 - Prob. 16.42EPCh. 16 - Prob. 16.43EPCh. 16 - Prob. 16.44EPCh. 16 - Prob. 16.45EPCh. 16 - Prob. 16.46EPCh. 16 - Prob. 16.47EPCh. 16 - Prob. 16.48EPCh. 16 - Prob. 16.49EPCh. 16 - Prob. 16.50EPCh. 16 - Prob. 16.51EPCh. 16 - Prob. 16.52EPCh. 16 - Prob. 16.53EPCh. 16 - Prob. 16.54EPCh. 16 - Prob. 16.55EPCh. 16 - Prob. 16.56EPCh. 16 - Give the IUPAC name for each of the following...Ch. 16 - Give the IUPAC name for each of the following...Ch. 16 - Prob. 16.59EPCh. 16 - Give the common name for each of the carboxylic...Ch. 16 - Prob. 16.61EPCh. 16 - Write a chemical equation for the preparation of...Ch. 16 - Prob. 16.63EPCh. 16 - Prob. 16.64EPCh. 16 - Prob. 16.65EPCh. 16 - Prob. 16.66EPCh. 16 - Prob. 16.67EPCh. 16 - Prob. 16.68EPCh. 16 - Prob. 16.69EPCh. 16 - Prob. 16.70EPCh. 16 - Prob. 16.71EPCh. 16 - Prob. 16.72EPCh. 16 - Prob. 16.73EPCh. 16 - Prob. 16.74EPCh. 16 - Prob. 16.75EPCh. 16 - Prob. 16.76EPCh. 16 - Prob. 16.77EPCh. 16 - Prob. 16.78EPCh. 16 - Prob. 16.79EPCh. 16 - Prob. 16.80EPCh. 16 - Prob. 16.81EPCh. 16 - Prob. 16.82EPCh. 16 - Prob. 16.83EPCh. 16 - Prob. 16.84EPCh. 16 - Prob. 16.85EPCh. 16 - Prob. 16.86EPCh. 16 - Prob. 16.87EPCh. 16 - Prob. 16.88EPCh. 16 - Prob. 16.89EPCh. 16 - Prob. 16.90EPCh. 16 - Prob. 16.91EPCh. 16 - Prob. 16.92EPCh. 16 - Assign an IUPAC name to each of the following...Ch. 16 - Prob. 16.94EPCh. 16 - Prob. 16.95EPCh. 16 - Prob. 16.96EPCh. 16 - Prob. 16.97EPCh. 16 - Prob. 16.98EPCh. 16 - Prob. 16.99EPCh. 16 - Prob. 16.100EPCh. 16 - How many carbon atoms are present in a molecule of...Ch. 16 - Prob. 16.102EPCh. 16 - Prob. 16.103EPCh. 16 - Prob. 16.104EPCh. 16 - Prob. 16.105EPCh. 16 - Prob. 16.106EPCh. 16 - Prob. 16.107EPCh. 16 - Prob. 16.108EPCh. 16 - Prob. 16.109EPCh. 16 - Prob. 16.110EPCh. 16 - Prob. 16.111EPCh. 16 - Prob. 16.112EPCh. 16 - Prob. 16.113EPCh. 16 - Prob. 16.114EPCh. 16 - Prob. 16.115EPCh. 16 - Prob. 16.116EPCh. 16 - Prob. 16.117EPCh. 16 - Prob. 16.118EPCh. 16 - Prob. 16.119EPCh. 16 - Prob. 16.120EPCh. 16 - Prob. 16.121EPCh. 16 - Prob. 16.122EPCh. 16 - Prob. 16.123EPCh. 16 - Prob. 16.124EPCh. 16 - Prob. 16.125EPCh. 16 - Prob. 16.126EPCh. 16 - Prob. 16.127EPCh. 16 - Prob. 16.128EPCh. 16 - Prob. 16.129EPCh. 16 - Prob. 16.130EPCh. 16 - Prob. 16.131EPCh. 16 - Prob. 16.132EPCh. 16 - Prob. 16.133EPCh. 16 - Prob. 16.134EPCh. 16 - Prob. 16.135EPCh. 16 - Prob. 16.136EPCh. 16 - Prob. 16.137EPCh. 16 - Prob. 16.138EPCh. 16 - Prob. 16.139EPCh. 16 - Prob. 16.140EPCh. 16 - Prob. 16.141EPCh. 16 - Prob. 16.142EPCh. 16 - Prob. 16.143EPCh. 16 - Prob. 16.144EPCh. 16 - Prob. 16.145EPCh. 16 - Prob. 16.146EPCh. 16 - Prob. 16.147EPCh. 16 - Prob. 16.148EPCh. 16 - Draw a condensed structural formula for the...Ch. 16 - Prob. 16.150EPCh. 16 - Prob. 16.151EPCh. 16 - Prob. 16.152EPCh. 16 - Prob. 16.153EPCh. 16 - Prob. 16.154EPCh. 16 - Prob. 16.155EPCh. 16 - Prob. 16.156EPCh. 16 - Prob. 16.157EPCh. 16 - Prob. 16.158EPCh. 16 - Prob. 16.159EPCh. 16 - Prob. 16.160EPCh. 16 - Prob. 16.161EPCh. 16 - Prob. 16.162EPCh. 16 - Prob. 16.163EPCh. 16 - Prob. 16.164EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forwardWhat is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Stuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward个 Stuc X ctclix ALE X A ALE × A ALE X Lab x (195 × Nut x M Inbx EF 目 → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpz Chapter 12 HW = Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited Part: 1/2 Part 2 of 2 Give the IUPAC name. Check 3 50°F Clear ©2025 McGraw Hill L Q Search webp a عالياكarrow_forward
- 个 Stuck x ctc xALE X A ALE × A ALE X Lab x (19: x - G www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-1Q1g8NUi-mObka ZLx2twjEhK7mVG6PUUIO06 Chapter 12 HW 三 Question 26 of 39 (4 points) 1 Question Attempt: 1 of Unlimited Answer the following questions about the given alkane. Part: 0 / 2 Part 1 of 2 Give the IUPAC name. Skip Part 2 53°F Clear Check × Q Search hp hp 02arrow_forwardCalculate the equilibrium constant at 25.0 oC for the following equation. Cd(s) + Sn+2(aq) ↔Cd+2(aq) + Sn(s) Group of answer choices 3.11x104 1.95x1018 9.66x108 1.40x109arrow_forwardWhat is the pH at the cathode for the following cell written in line notation at 25.0 oC with a Ecell = -0.2749 V? Ni(s)|Ni+2(aq, 1.00 M)||H+1(aq, ?M)|H2(g, 1.00 atm)|Pt(s)arrow_forward
- Calculate Ecell for a hydrogen fuel cell at 95.0 oC using the following half-reactions with PH2 = 25.0 atm and PO2 = 25.0 atm. O2(g) + 4H+1(aq) + 4e-1 → 2H2O(l) Eo = 1.229 V 2H2(g) → 4H+1(aq) + 4e-1 Eo = 0.00 Varrow_forwardCalculate Ecell at 25.0 oC using the following half-reactions with [Ag+1] = 0.0100 M and [Sn+2] = 0.0200 M. Ag+1(aq) + 1e-1 Ag(s) Sn+2(aq) + 2e-1 Sn(s)arrow_forwardDone 18:19 www-awu.aleks.com Chapter 12 HW Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited .. LTE סוי 9 ✓ 20 ✓ 21 × 22 23 24 25 26 27 28 29 30 Answer the following questions about the given alkane. Part: 0 / 2 Part 1 of 2 Classify each carbon atom as a 1º, 2º, 3º, or 4°. Highlight in red any 1° carbons, highlight in blue any 2° carbons. highlight in green any 3° carbons, and leave any 4° carbons unhighlighted. Skip Part Check Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility ☑ คarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,