
(a)
Interpretation:
IUPAC name for the acrylic acid has to be given.
Concept Introduction:
For naming a
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for
aldehyde , the carboxyl carbon is always numbered 1. - The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl
functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix. - If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 16.33EP
IUPAC name of acrylic acid is propenoic acid.
Explanation of Solution
Structure of acrylic acid is,
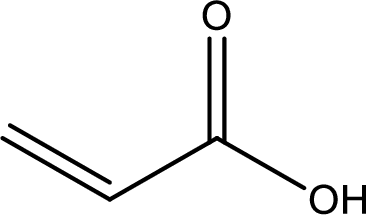
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The structure contains a double bond between carbon atoms. Hence, the parent is propene. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propenoic acid.
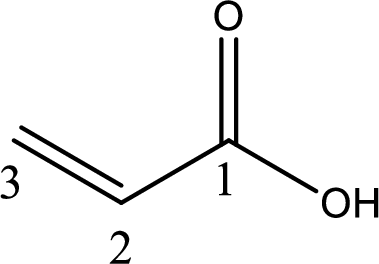
Looking for substituents it is found that there are no substituents present on the carbon chain. Hence, the IUPAC name of the acrylic acid is propenoic acid.
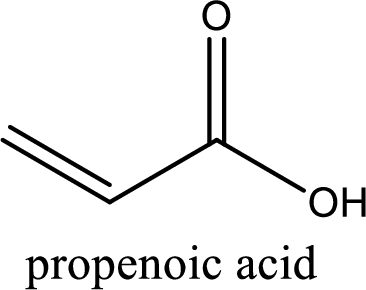
IUPAC name of acrylic acid is given.
(b)
Interpretation:
IUPAC name for the lactic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(b)
Answer to Problem 16.33EP
IUPAC name of lactic acid is 2-hydroxypropanoic acid.
Explanation of Solution
Structure of lactic acid is,
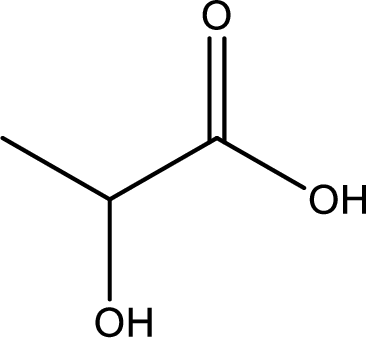
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The parent alkane is propane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propanoic acid.
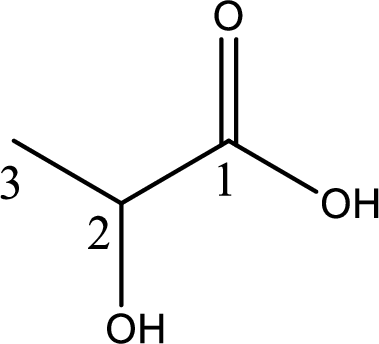
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the lactic acid is 2-hydroxypropanoic acid.
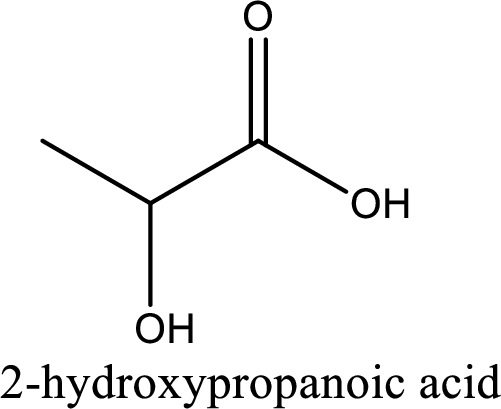
IUPAC name of lactic acid is given.
(c)
Interpretation:
IUPAC name for the maleic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(c)
Answer to Problem 16.33EP
IUPAC name of maleic acid is cis-butenedioic acid.
Explanation of Solution
Structure of maleic acid is,
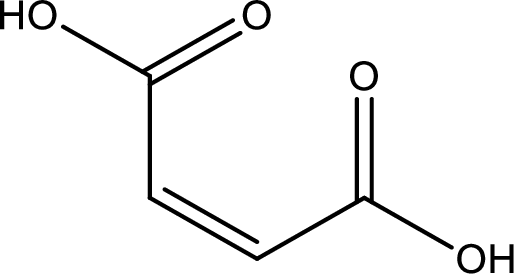
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The structure contains a double bond in it. The parent carbon chain is butene. The given structure contains two carboxyl groups. The carboxylic acid is named by adding suffix “-dioic acid”. This gives the name of carboxylic acid as butenedioic acid.
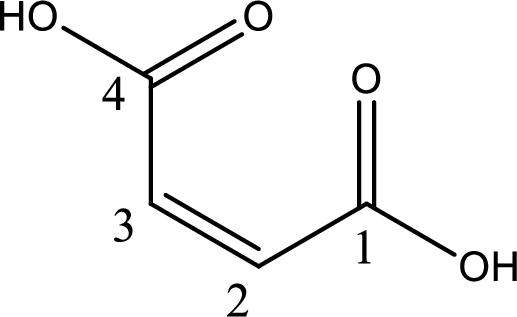
Looking for substituents it is found that there are no substituents present in the carbon chain. Stereochemistry is possible across the double bond. As the two hydrogen atoms are on the same side of double bond, the configuration at the double bond is “cis”. This has to be included in the name to get the IUPAC name. IUPAC name of the maleic acid is found as cis-butenedioic acid.
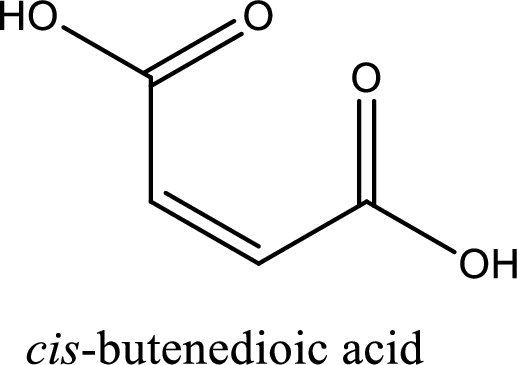
IUPAC name of maleic acid is given.
(d)
Interpretation:
IUPAC name for the glycolic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(d)
Answer to Problem 16.33EP
IUPAC name of glycolic acid is 2-hydroxyethanoic acid.
Explanation of Solution
Structure of glycolic acid is,
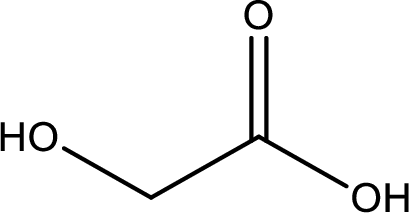
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a two carbon chain. The parent alkane is ethane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as ethanoic acid.
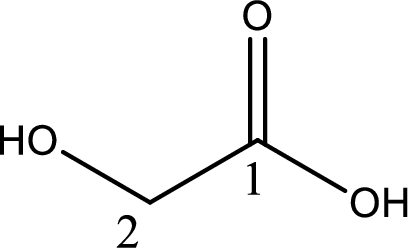
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the glycolic acid is 2-hydroxyethanoic acid.
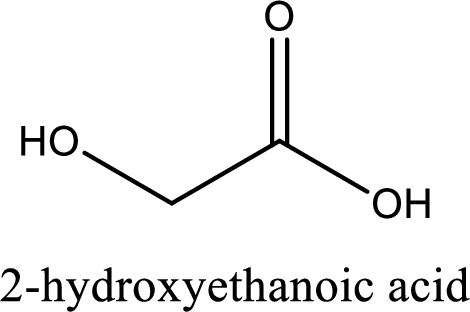
IUPAC name of glycolic acid is given.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





