
(a)
Interpretation:
Acrylic acid has to be classified among the given options.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
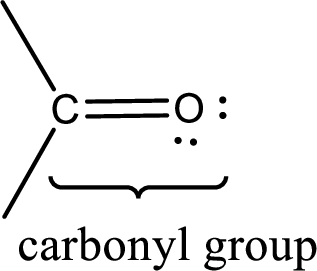
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
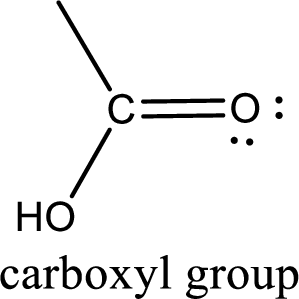
Apart from carboxyl group, there can be other functional group also that is present in the
(b)
Interpretation:
Maleic acid has to be classified among the given options.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
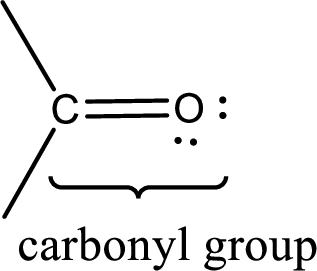
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
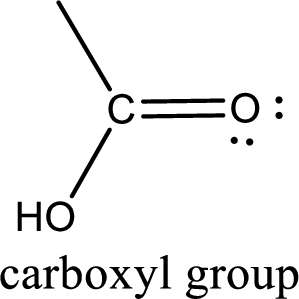
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
(c)
Interpretation:
Succinic acid has to be classified among the given options.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
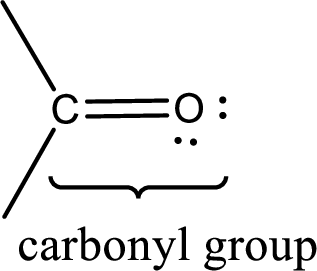
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
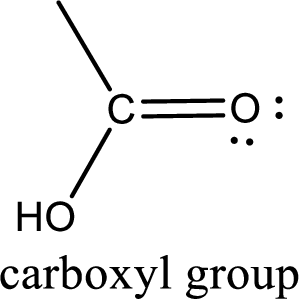
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
(d)
Interpretation:
Caproic acid has to be classified among the given options.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
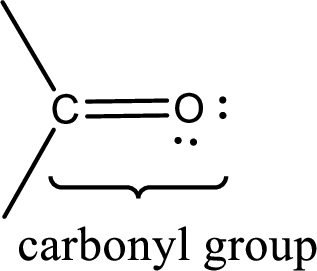
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
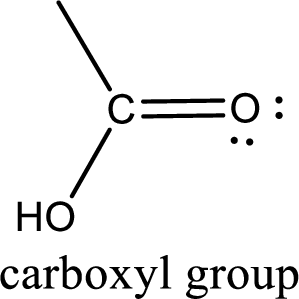
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry
- State three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forwardPrimary and global quantum yields in photochemistry. Define them and give their formulas. Differentiate between them.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





