
Concept explainers
(a)
Interpretation:
The olecular structure of heptanes must be drawn.
Concept Introduction:
Heptane is a straight chain hydrocarbon with 7 carbons.
(a)
Answer to Problem SIII4RE
Molecular structure of heptane is drawn below.
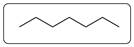
Explanation of Solution
In the molecular structure of heptanes only carbon chain is shown. Hydrogens are removed for simplicity. There are total 7 carbon and 16 hydrogen atoms. All the carbon is single bonded with another 4 atoms out of which two C and two hydrogen atoms.
b)
Interpretation:
Combustion reaction of heptane must be given.
Concept Introduction:
Hydrocarbons undergo combustion reaction to produce carbon dioxide and water as shown below.
Here x and y represents number of carbon and hydrogen atoms in the hydrocarbon.
b)
Answer to Problem SIII4RE
Explanation of Solution
There are 7 carbons and 16 hydrogens. So from 1 mole heptanes 7 moles of carbon dioxide and 11 moles of water were obtained after complete combustion.
c)
Interpretation:
Using average bond energies, the heat of reaction of heptanes must be calculated.
Concept Introduction:
Heat of reaction is the difference of energies required to break the bonds and energy obtained after bonds formation.
c)
Answer to Problem SIII4RE
Heat of reaction for the above combustion reaction is -4332 kJ/mol.
Explanation of Solution
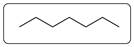
In the combustion reaction of heptanes 6 C-C, 16 C-H and 11 O=O bonds are broken. 14 C=O bonds and 16 O-H bonds are produced.
These bond energies must be considered to calculate the heat of reaction for the above combustion reaction.
Bond energies of C-C, C-H and O=O bonds are 346, 413 and 498 kJ/mol respectively.
Bond energies of C=O and O-H are 799 and 463 kJ/mol respectively.
Energy required to break 6 C-C, 16 C-H and 11 O=O bonds can be calculated as follows:
Energy obtained for the formation of 14 C=O bonds and 16 O-H bonds can be calculated as follows:
Heat of reaction
Chapter U5 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Microbiology: An Introduction
Biology: Life on Earth (11th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Campbell Biology (11th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forwardCorrect each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forward
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





