
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.SE, Problem 55AP
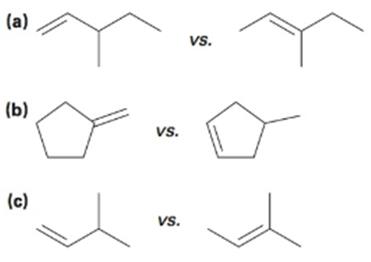
Use Hammond’s Postulate to determine which
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major product of the following reaction and then draw a curved arrow mechanism for its formation.
Part: 0/2
Part 1 of 2
H₂SO
heat
: OH
90
Draw the structure of the major product.
Click and drag to start drawing a
structure.
3
Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all
nonzero formal charges.
C
Ö-H
H
+
-S-OH
.0.
Add/Remove step
X
टे
Click and drag to start
drawing a structure.
Draw a curved arrow mechanism for its formation. You may need to re-draw structures to show certain bonds. Ensure that HSO is used as the base to
deprotonate the ẞ carbon when necessary.
C
HO
: OH
HO: OH
=s
=
+
1
Add/Remove step
X
Click and drag to start
drawing a structure.
Chapter 7 Solutions
Organic Chemistry
Ch. 7.2 - Calculate the degree of unsaturation in each of...Ch. 7.2 - Prob. 2PCh. 7.2 - Diazepam, marketed as an antianxiety medication...Ch. 7.3 - Give IUPAC names for the following compounds:Ch. 7.3 - Prob. 5PCh. 7.3 - Prob. 6PCh. 7.3 - Prob. 7PCh. 7.4 - Prob. 8PCh. 7.4 - Prob. 9PCh. 7.4 - Prob. 10P
Ch. 7.5 - Which member in each of the following sets ranks...Ch. 7.5 - Prob. 12PCh. 7.5 - Prob. 13PCh. 7.5 - Prob. 14PCh. 7.6 - Prob. 15PCh. 7.8 - Prob. 16PCh. 7.8 - Prob. 17PCh. 7.9 - Show the structures of the carbocation...Ch. 7.9 - Draw a skeletal structure of the following...Ch. 7.10 - Prob. 20PCh. 7.11 - On treatment with HBr, vinylcyclohexane undergoes...Ch. 7.SE - Prob. 22VCCh. 7.SE - Prob. 23VCCh. 7.SE - The following carbocation is an intermediate in...Ch. 7.SE - Prob. 25VCCh. 7.SE - Predict the major product and show the complete...Ch. 7.SE - Prob. 27MPCh. 7.SE - When 1, 3-butadiene reacts with one mole of HBr,...Ch. 7.SE - When methyl vinyl ether reacts with a strong acid,...Ch. 7.SE - Addition of HCl to 1-isopropylcyclohexene yields a...Ch. 7.SE - Addition of HCl to...Ch. 7.SE - Limonene, a fragrant hydrocarbon found in lemons...Ch. 7.SE - Prob. 33MPCh. 7.SE - Calculate the degree of unsaturation in the...Ch. 7.SE - Prob. 35APCh. 7.SE - Prob. 36APCh. 7.SE - Name the following alkenes:Ch. 7.SE - Draw structures corresponding to the following...Ch. 7.SE - Prob. 39APCh. 7.SE - Prob. 40APCh. 7.SE - Prob. 41APCh. 7.SE - Prob. 42APCh. 7.SE - Prob. 43APCh. 7.SE - Draw and name the 17 alkene isomers, C6H12,...Ch. 7.SE - Prob. 45APCh. 7.SE - Prob. 46APCh. 7.SE - Which of the following E, Z designations are...Ch. 7.SE - Prob. 48APCh. 7.SE - trans-2-Butene is more stable than cis-2-butene by...Ch. 7.SE - Prob. 50APCh. 7.SE - Normally, a trans alkene is more stable than its...Ch. 7.SE - trans-Cyclooctene is less stable than...Ch. 7.SE - Prob. 53APCh. 7.SE - Prob. 54APCh. 7.SE - Use Hammond’s Postulate to determine which...Ch. 7.SE - Prob. 56APCh. 7.SE - Predict the major product in each of the following...Ch. 7.SE - Prob. 58APCh. 7.SE - Prob. 59APCh. 7.SE - Prob. 60APCh. 7.SE - Allene (1,2-propadiene), H2C=C=CH2, has two...Ch. 7.SE - The heat of hydrogenation for allene (Problem...Ch. 7.SE - Retin A, or retinoic acid, is a medication...Ch. 7.SE - Prob. 64APCh. 7.SE - tert-Butyl esters [RC02C(CH3)3] are converted into...Ch. 7.SE - Vinylcyclopropane reacts with HBr to yield a...Ch. 7.SE - Prob. 67APCh. 7.SE - Prob. 68APCh. 7.SE - Prob. 69APCh. 7.SE - Prob. 70APCh. 7.SE - Prob. 71APCh. 7.SE - Reaction of 2, 3-dimethyl-l-butene with HBr leads...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following could 1,2-ethanediol be directly synthesized from? OH HO О 0 0. O ?arrow_forwardDesign a synthesis of 1,2-diethoxyethane from an alkene. Select the single best answer for each part. Part: 0/3 Part 1 of 3 Which of the following could 1,2-diethoxyethane be directly synthesized from? O HO 0 HO.... OH HO HO × 5 > ?arrow_forwardDraw the skeletal structure of the major organic product of each step of the reaction sequence. Part: 0/2 Part 1 of 2 Part: 1/2 Part 2 of 2 Continue OH NaH Na Na Br + Click and drag to start drawing a structure. X : X G : Garrow_forward
- pleasearrow_forwardplease help me please pleasearrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N2 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm ☑ 5 00. 18 Ararrow_forward
- i need help with the followingarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO(g) +Cl₂ (g) = 2NOC1 (g) AGº = -41. kJ Now suppose a reaction vessel is filled with 8.90 atm of chlorine (C12) and 5.71 atm of nitrosyl chloride (NOC1) at 1075. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. atm ☑ 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY