
Indicate whether each of the following aspects of triacylglycerol digestion is associated with the (1) mouth, (2) stomach, (3) small intestine, (4) intestinal cells, or (5) bloodstream.
- a. Chyme is produced.
- b. Gastric lipases are active.
- c. Initial production of monoacylglycerols occurs.
- d. Fatty acid micelles are formed.
(a)
Interpretation: To identify whether the chyme is produced in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: In the stomach, the muscle walls of the stomach churn the food and other complex molecules into smaller particles. The churning action breaks up the lipid molecules into small droplets/globules which remains suspended on the other smaller components of the ingested food. This results in the formation of a semi-liquid material comprising of small lipid droplets, partly digested food and gastric secretions called chyme. The chyme, thereafter, resumes its journey to the small intestine.
Answer to Problem 14.1EP
Chyme production occurs in the stomach.
Explanation of Solution
Chyme is a semi-liquid material composed of small lipid droplets, partly digested food and gastric secretions. Since the initial breakdown of lipid molecules and activity of the gastric lipases begins in the stomach, hence the formation of chyme occurs in the stomach.
(b)
Interpretation: To identify whether the gastric lipase is active in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Gastric lipase is an acidic lipase secreted by the gastric chief cells in the fundic mucosa in the stomach. The function of the enzyme is to break down lipid molecules. Gastric lipases are enzymes which break down lipids.
Answer to Problem 14.1EP
Gastric lipases are active in the stomach.
Explanation of Solution
Gastric lipases are present in the stomach and help in the initial hydrolysis of the triacylglycerol molecules. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). Around 10% of the triacylglycerols are hydrolyzed in the stomach by the action of gastric lipases. Hence, gastric lipases are active in the stomach.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
(c)
Interpretation: To identify whether the initial production of monoacylglycerol occurs in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: The initial lipid digestion begins in the stomach in the presence of gastric lipases. Gastric lipases are enzymes which break down lipids. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. Here the digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
Answer to Problem 14.1EP
The initial production of monoacylglycerol occurs in the stomach.
Explanation of Solution
In the stomach, around 10% of the triacylglycerol is hydrolyzed into monoacylglycerol and free fatty acids under the activity of gastric lipases. The remaining hydrolysis occurs in the small intestine. Hence, the initial production of monoacylglycerol occurs in the stomach.
(d)
Interpretation: To identify whether the fatty acid micelles are formed in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
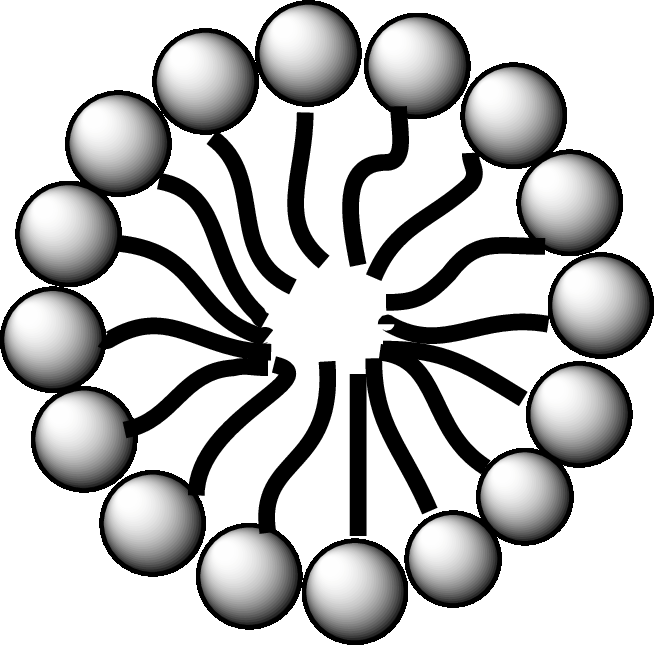
Concept introduction: Fatty acid is a carboxylic acid that contains a long hydrophobic hydrocarbon chain and a polar carboxyl group. The fatty acids are aligned such that the hydrophobic hydrocarbon chain is directed inwards away from the polar environment and the polar carboxyl group head is directed outwards. The aggregate thus formed is called micelle.
A general representation of fatty acid is,
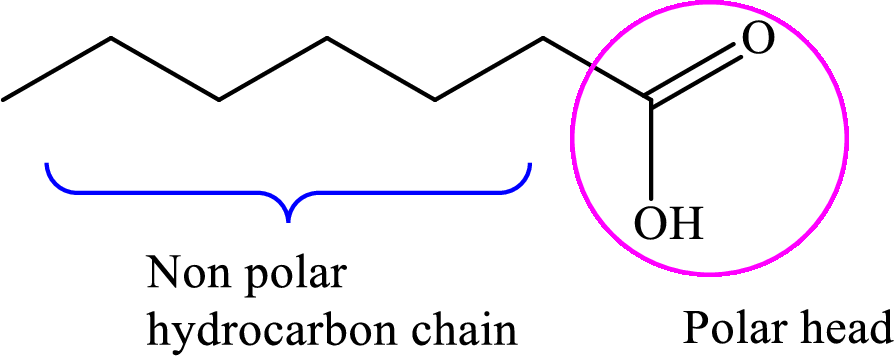
A diagrammatic representation of micelle is as follows:
Answer to Problem 14.1EP
Fatty acid micelles are formed in the small intestine.
Explanation of Solution
A fatty acid micelle is a spherical droplet which is primarily composed of free fatty acids, monoacylglycerols, and bile.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
In the small intestine, the monoacylglycerols and free fatty acids along with bile and pancreatic lipases, form micelles. Micelles are later absorbed by the cell membranes of the intestinal cells.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic And Biological Chemistry
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
General, Organic, and Biological Chemistry - 4th edition
Human Anatomy & Physiology (2nd Edition)
Anatomy & Physiology (6th Edition)
Biology: Concepts and Investigations
- A 25.0 g sample of water was cooled from 23.9°C to 12.7°C, how much heat was released? (Assume thatthe specific heat of water is 4.18 J/g °C)arrow_forwardZeolites: environmental applications.arrow_forward" is The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 best described as a hybrid of several contributing resonance forms, two of which are shown here. HO :0: :Ö: HO + Bicarbonate is crucial for the control of body pH (for example, blood pH: 7.4). A more self-indulgent use is in baking soda, where it serves as a source of CO2 CO₂ 2 gas, which gives bread and pastry their fluffy constituency. (i) Draw at least one additional resonance form. = (ii) Using curved "electron-pushing" arrows, show how these Lewis structures may be interconverted by movement of electron pairs. (iii) Determine which form or forms will be the major contributor(s) to the real structure of bicarbonate, explaining your answer on the basis of the criteria in Section 1-5.arrow_forward
- Which of these is the best use of a volumetric flask? measuring how much liquid it contains delivering a precise amount of liquid to another container holding solutions making solutions of precise concentrationarrow_forwardYou're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forward
- Is molecule 6 an enantiomer?arrow_forwardShow work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forward
- Show work. don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





