
Concept explainers
(a)
Interpretation:
The intermediate generated in the first cycle of the lipogenesis pathway that has a carbon chain that is derived from a C4 keto monoacid has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The cyclic process occurs in the enzyme fatty acid synthase. The one turn of this cyclic process constitutes four reactions. The various intermediates formed in the process are associated with a carrier protein known as ACP.
Keto monoacid is the compound that has a keto group and
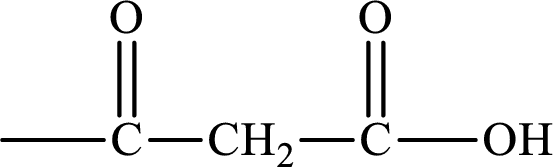
(b)
Interpretation:
The intermediate generated in the first cycle of the lipogenesis pathway that has a carbon chain that is derived from a C4 unsaturated monoacid has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The cyclic process occurs in the enzyme fatty acid synthase. The one turn of this cyclic process constitutes four reactions. The various intermediates formed in the process are associated with a carrier protein known as ACP.
Unsaturated monoacid is the compound that has double bond or triple bond and carboxylic acid group in its structure. The structure of unsaturated monoacid is:
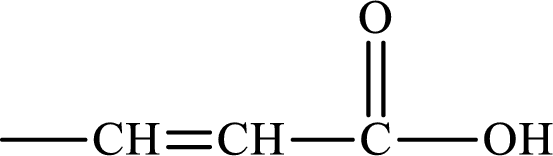
(c)
Interpretation:
The intermediate generated in the first cycle of the lipogenesis pathway that has a carbon chain that is produced by a dehydration reaction has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The cyclic process occurs in the enzyme fatty acid synthase. The one turn of this cyclic process constitutes four reactions. The various intermediates formed in the process are associated with a carrier protein known as ACP.
Dehydration reaction is the reaction in which water molecule is eliminated with the formation of the product.
(d)
Interpretation:
The intermediate generated in the first cycle of the lipogenesis pathway that has a carbon chain that is produced in a reaction that also produces CO2 to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The cyclic process occurs in the enzyme fatty acid synthase. The one turn of this cyclic process constitutes four reactions. The various intermediates formed in the process are associated with a carrier protein known as ACP.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic And Biological Chemistry
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forwardHow many grams of solid NaCN have to be added to 1.5L of water to dissolve 0.18 mol of Fe(OH)3 in the form Fe(CN)63 - ? ( For simplicity, ignore the reaction of CN - ion with water) Ksp for Fe(OH)3 is 2.8E -39, and Kform for Fe(CN)63 - is 1.0E31arrow_forward
- Draw the most stable chair conformation of 1-ethyl-1-methylcyclohexane, clearly showing the axial and equatorial substituents. [4] Draw structures corresponding to the following IUPAC name for each of the following compounds; [5] i) 4-Isopropyl-2,4,5-trimethylheptane ii) trans-1-tert-butyl-4-ethylcyclohexane iii) Cyclobutylcycloheptane iv) cis-1,4-di-isopropylcyclohexane (chair conformation) v) 3-Ethyl-5-isobutylnonanearrow_forwardDraw and name molecules that meet the following descriptions; [4] a) An organic molecule containing 2 sp2 hybridised carbon and 1 sp-hybridised carbon atom. b) A cycloalkene, C7H12, with a tetrasubstituted double bond. Also answer question 2 from the imagearrow_forwardH 14. Draw the line angle form of the following molecule make sure you use the proper notation to indicate spatial positioning of atoms. F F H 15. Convert the following condensed form to line angle form: (CH3)3CCH2COCH2CON(CH2CH3)2arrow_forward
- In a reaction between two reactants A and B, the half-life is the same for both only if(A) the stoichiometry A:B is 1:1.(B) the stoichiometry A:B is 1:2 or 2:1.arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


