
Concept explainers
(a)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
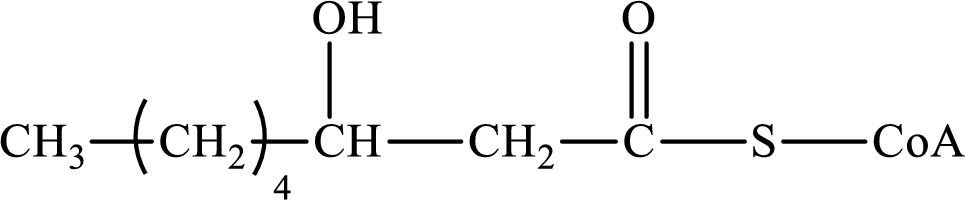
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
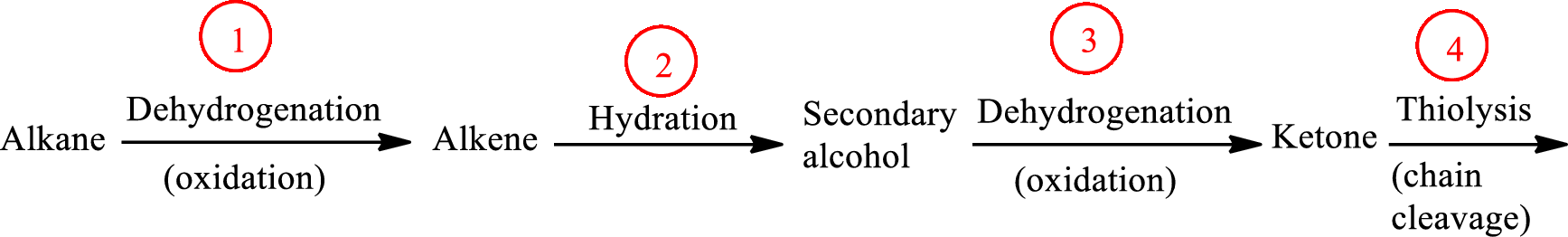
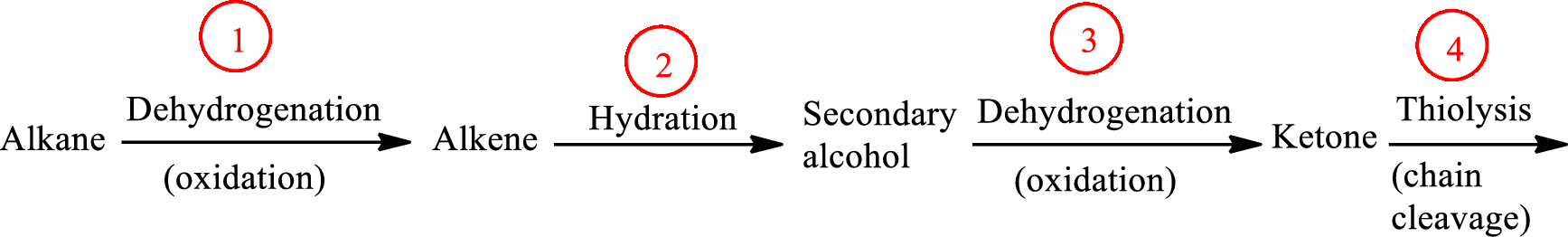
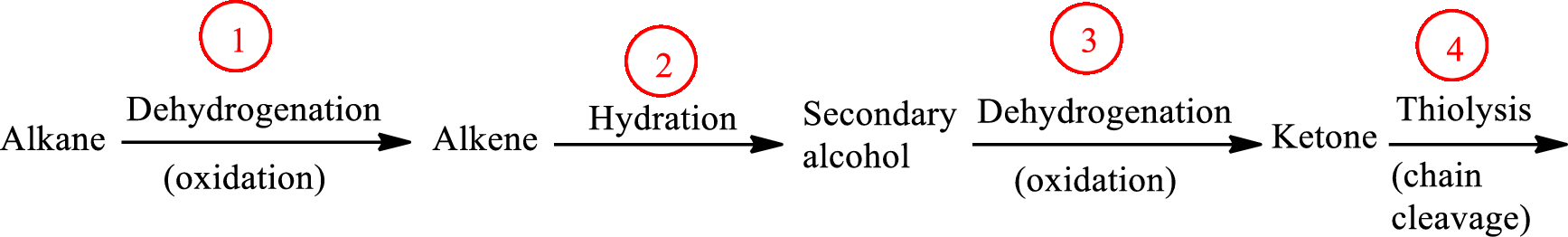
The functional group change in the β-oxidation pathway is as follows:
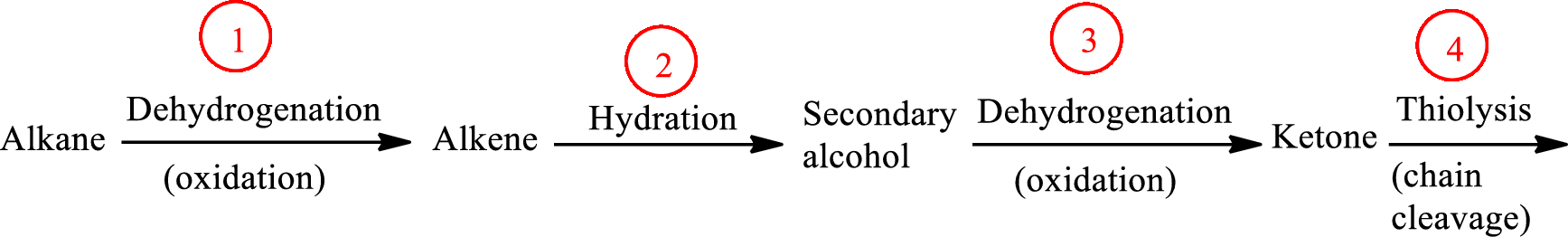
Here, R and R’ represent an alkyl group. In
(b)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
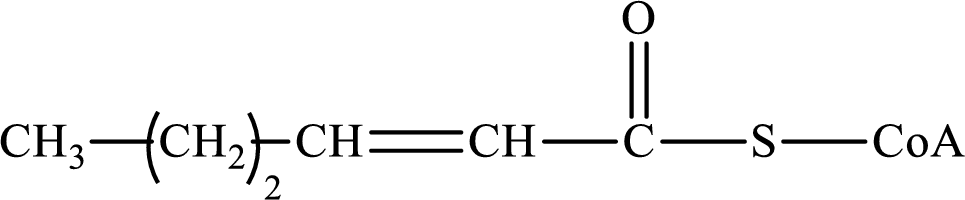
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
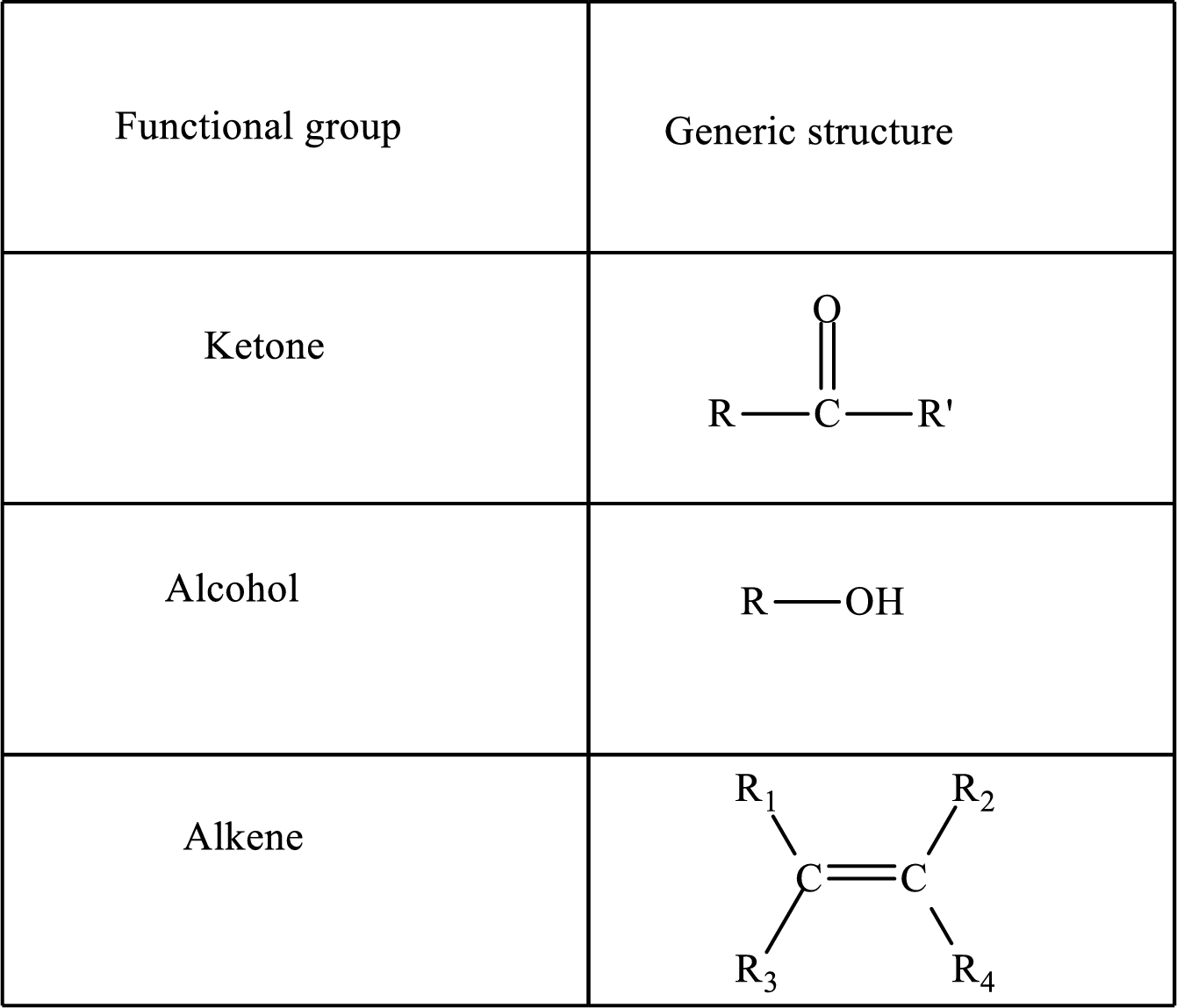
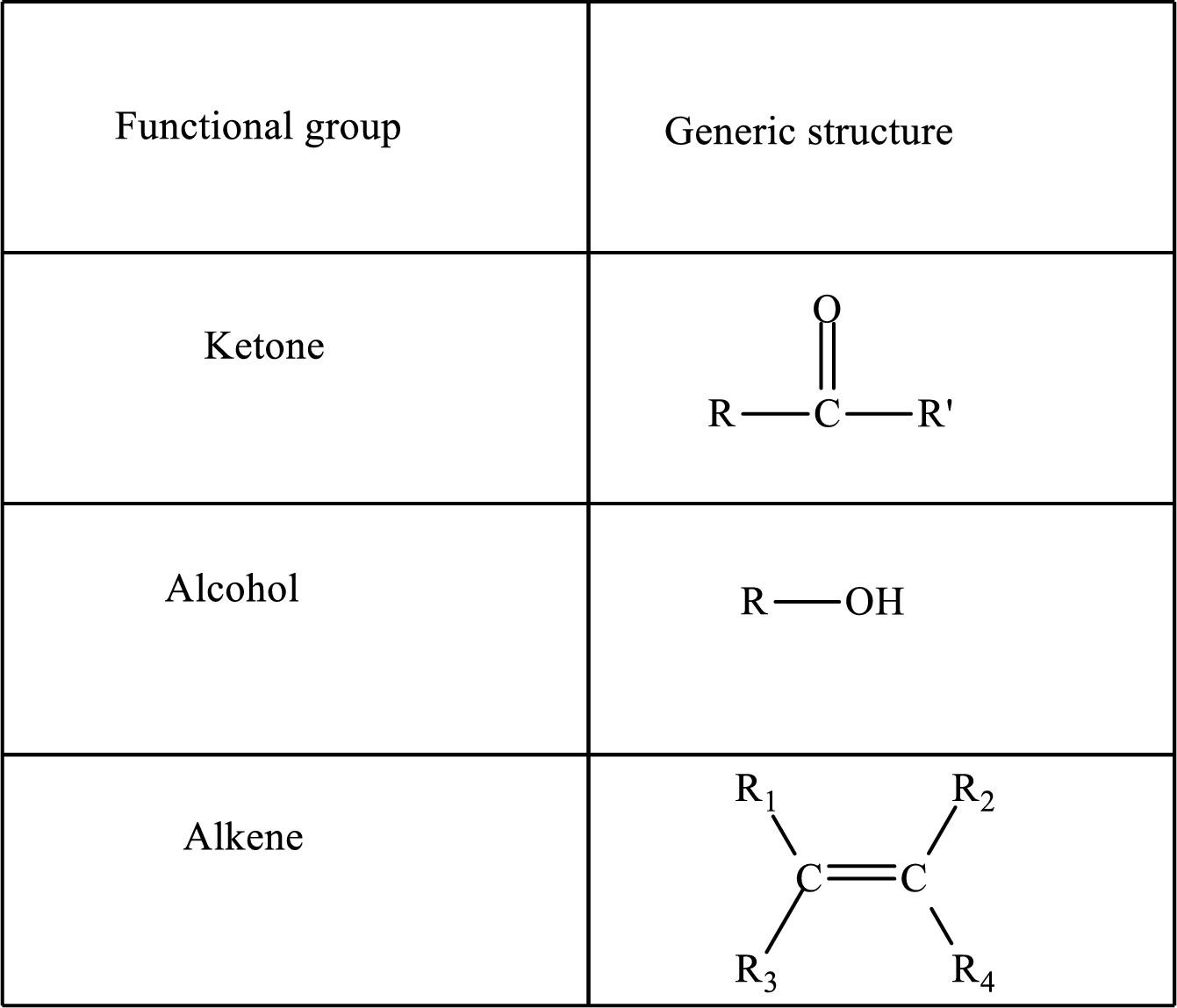
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
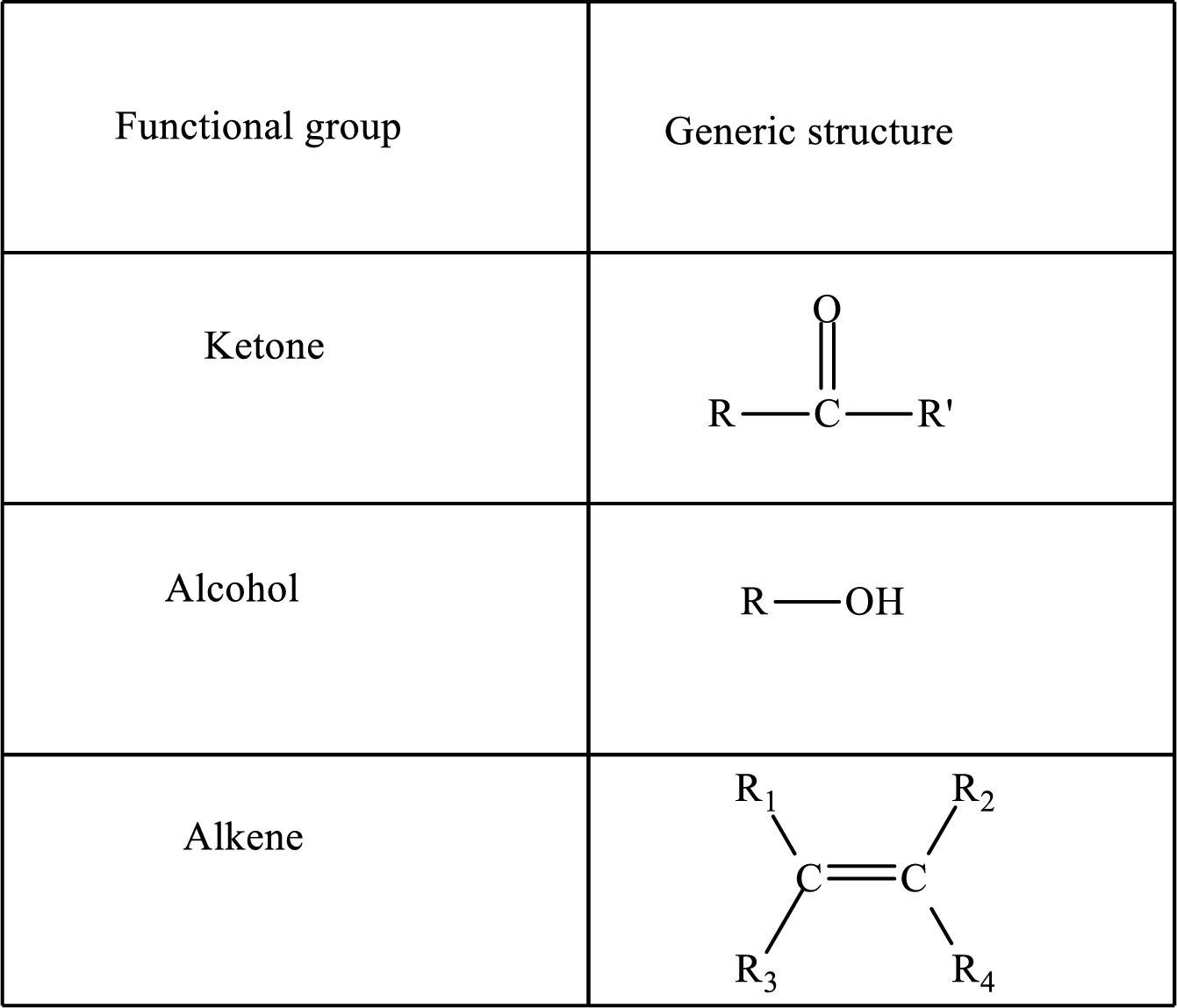
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(c)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
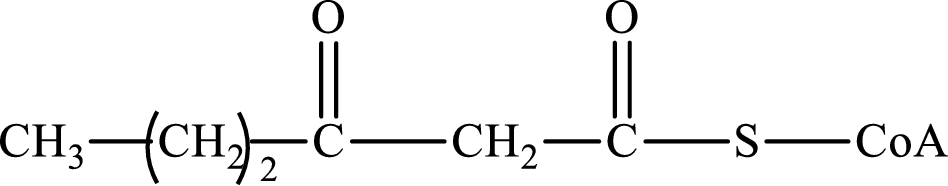
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
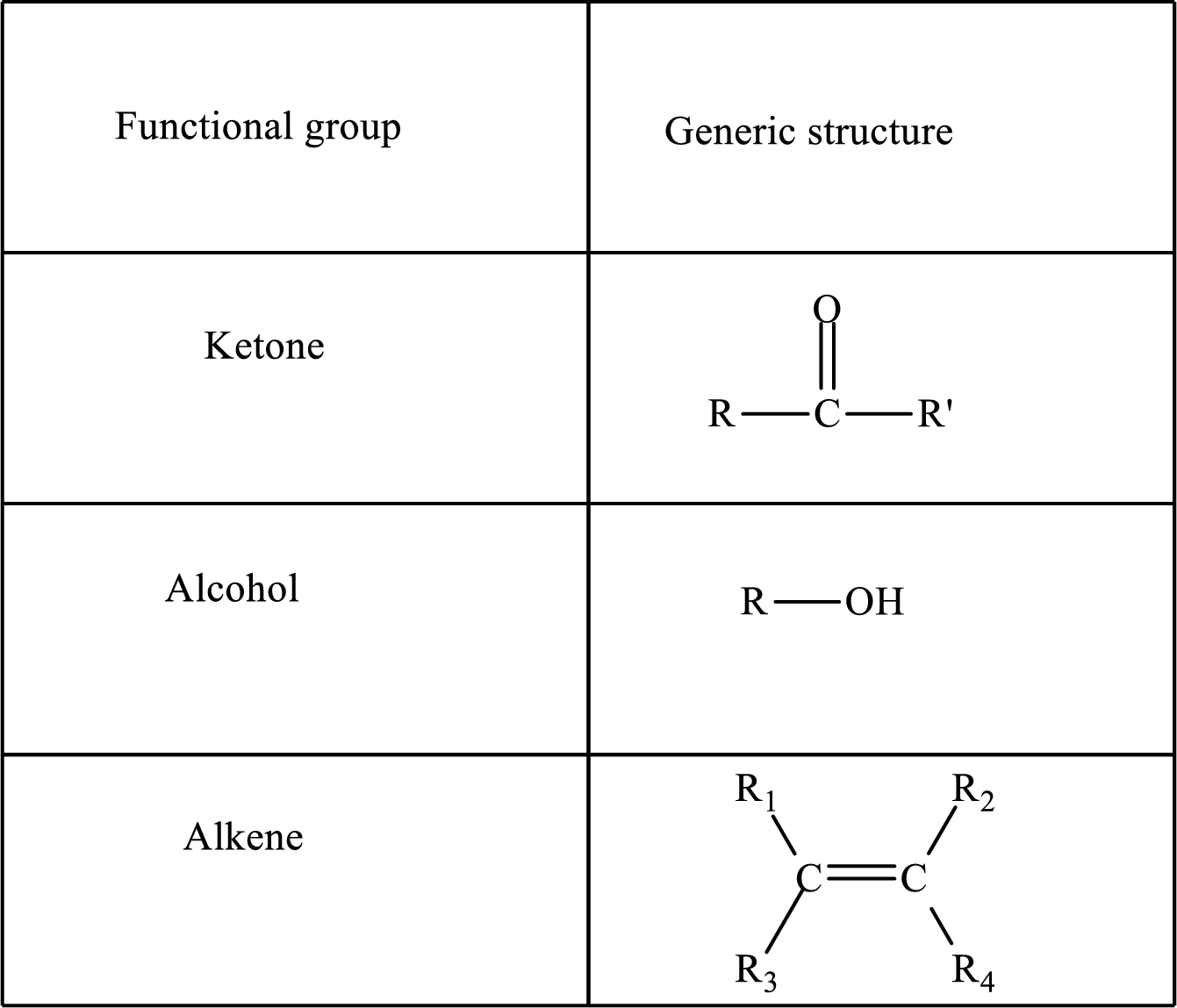
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(d)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
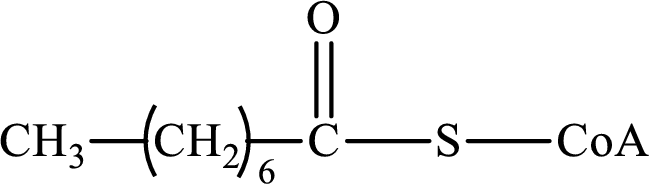
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic And Biological Chemistry
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





