
Concept explainers
(a)
Interpretation:
Whether “
Concept Introduction:
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions.
The
The first stage of fatty acid oxidation is the activation of fatty acids in the outer mitochondrial membrane. The fatty acid is activated by
(a)
Answer to Problem 14.25EP
Explanation of Solution
The enzymes that are needed for the oxidation of fatty acid are located in the mitochondrial matrix. Acyl CoA cannot pass through the inner mitochondrial membrane to the mitochondrial matrix because it is too large. A shuttle mechanism that involves the molecule carnitine effects the entry of Acyl CoA into the mitochondrial matrix.
An overview of the transportation of Acyl CoA in the carnitine shuttle system associated with the
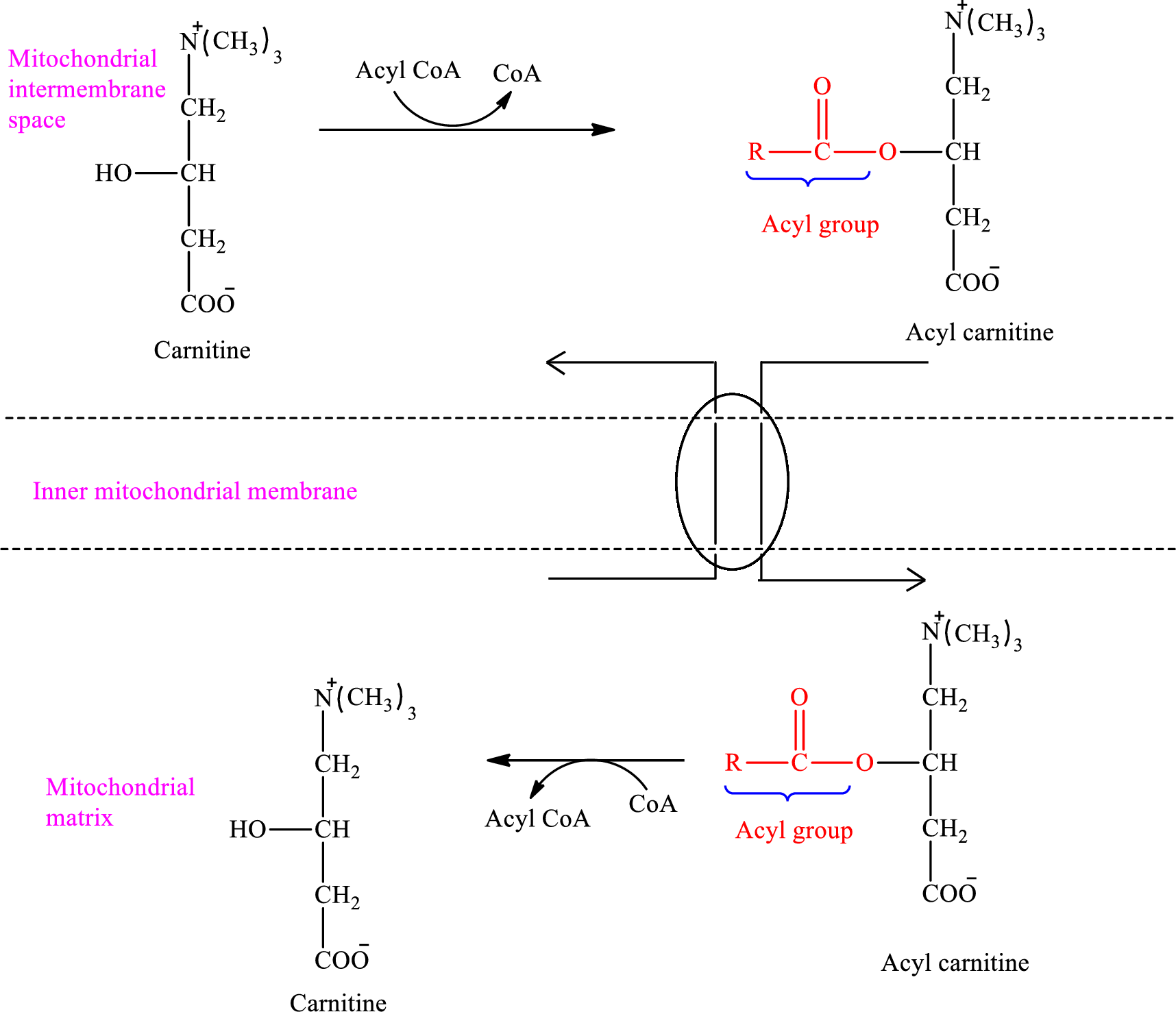
Therefore, Acyl CoA is encountered as a reactant in the intermembrane space.
(b)
Interpretation:
Whether “carnitine enters the inner mitochondrial membrane” in the mitochondrial matrix or in the mitochondrial intermembrane space in the carnitine shuttle system associated with the
Concept Introduction:
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions.
The
The first stage of fatty acid oxidation is the activation of fatty acids in the outer mitochondrial membrane. The fatty acid is activated by
(b)
Answer to Problem 14.25EP
Carnitine enters the inner mitochondrial membrane in the mitochondrial matrix.
Explanation of Solution
The enzymes that are needed for the oxidation of fatty acid are located in the mitochondrial matrix.
An overview of the transportation of
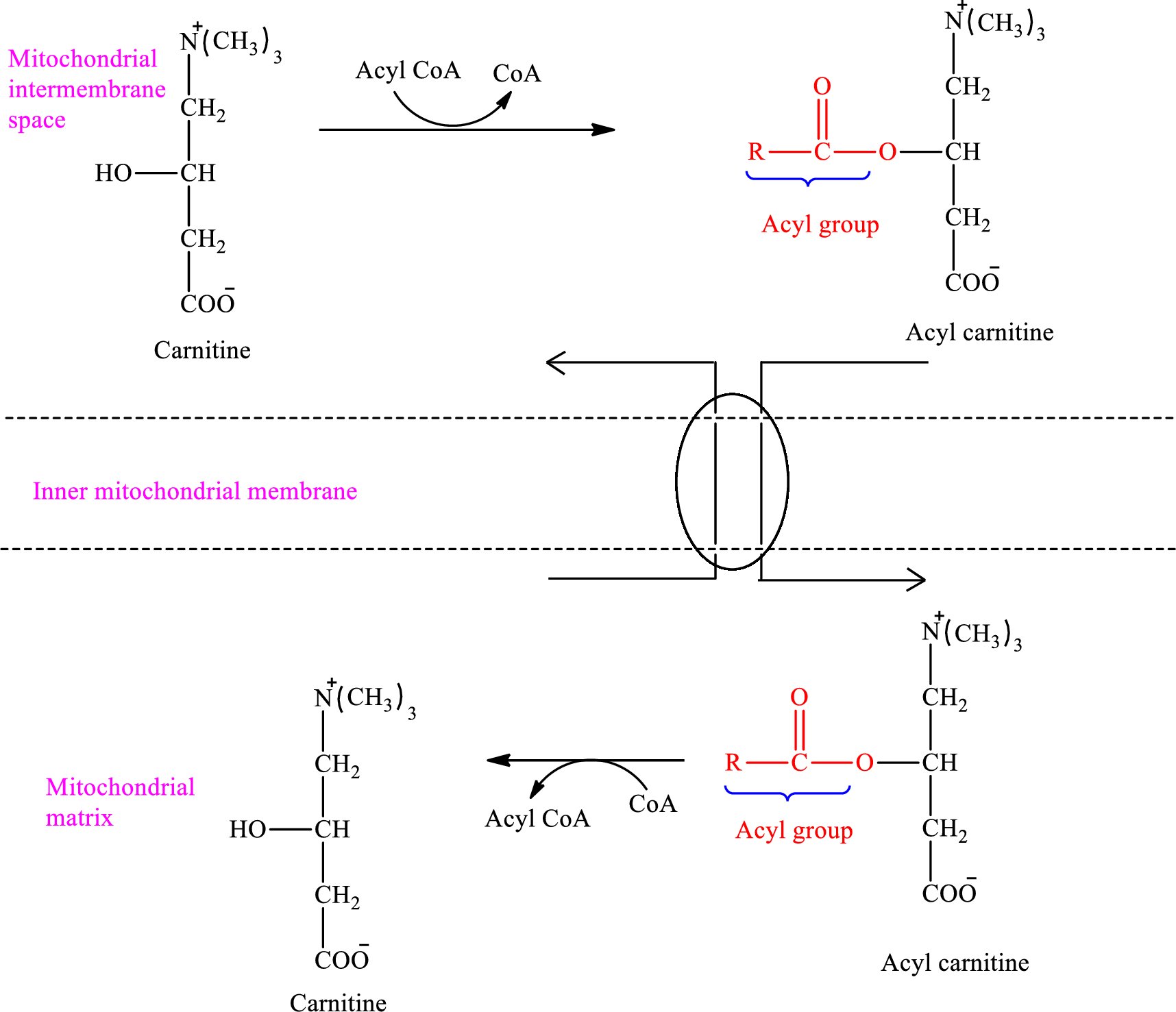
Carnitine molecule shuttles the activated fatty acid molecules across the inner mitochondrial membrane. Therefore, carnitine enters the inner mitochondrial membrane in the mitochondrial matrix.
(c)
Interpretation:
Whether “carnitine is converted to acyl carnitine” in the mitochondrial matrix or in the mitochondrial intermembrane space in the carnitine shuttle system associated with the
Concept Introduction:
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions.
The
The first stage of fatty acid oxidation is the activation of fatty acids in the outer mitochondrial membrane. The fatty acid is activated by
The enzymes that are needed for the oxidation of fatty acid are located in the mitochondrial matrix.
(c)
Answer to Problem 14.25EP
Carnitine is converted to acyl carnitine in the mitochondrial intermembrane space.
Explanation of Solution
Acyl group present in
An overview of the transportation of
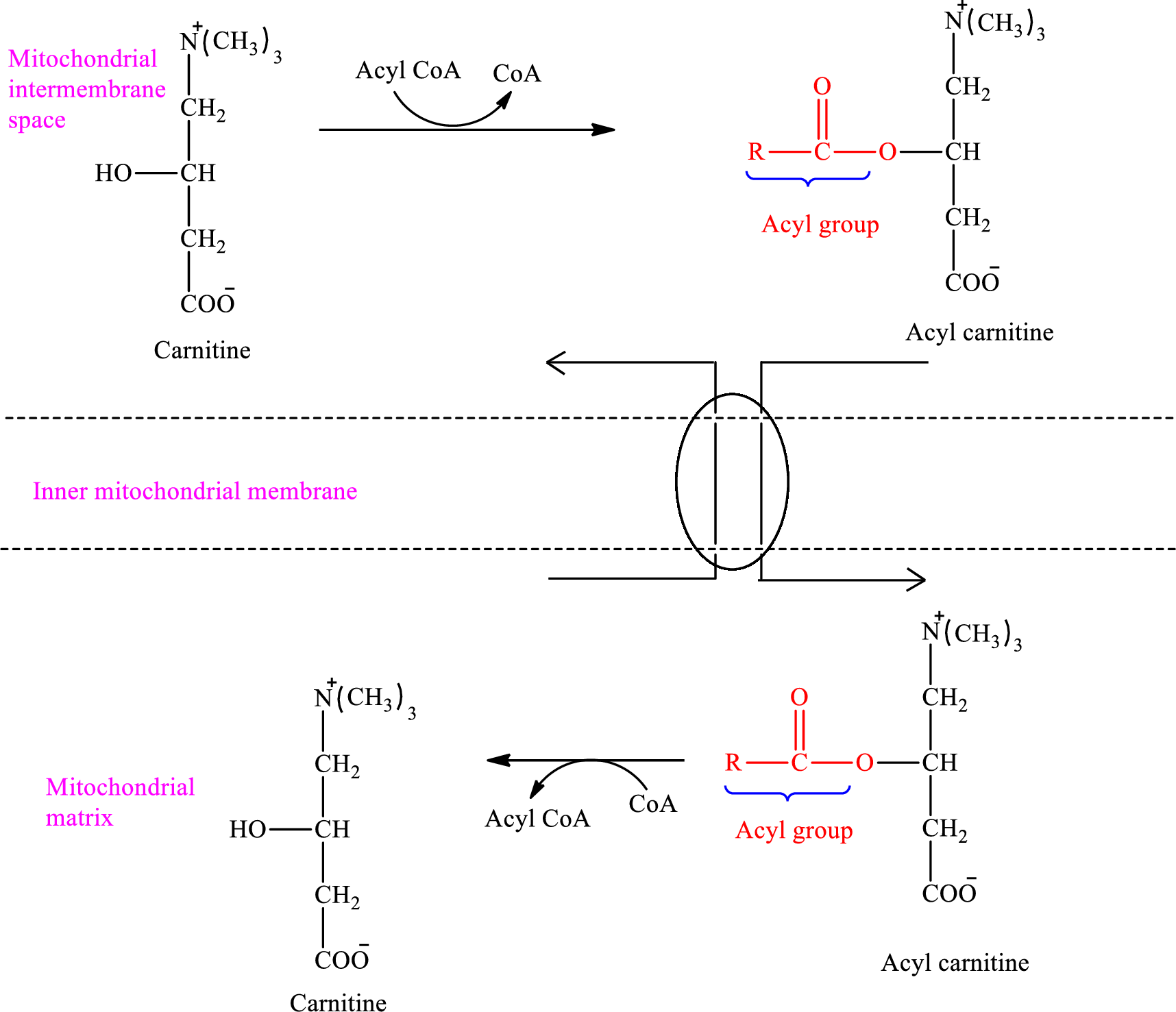
Therefore, carnitine is converted to acyl carnitine in the mitochondrial intermembrane space.
(d)
Interpretation:
Whether “free
Concept Introduction:
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions.
The
The first stage of fatty acid oxidation is the activation of fatty acids in the outer mitochondrial membrane. The fatty acid is activated by
The enzymes that are needed for the oxidation of fatty acid are located in the mitochondrial matrix.
(d)
Answer to Problem 14.25EP
Free
Explanation of Solution
Acyl group present in
An overview of the transportation of
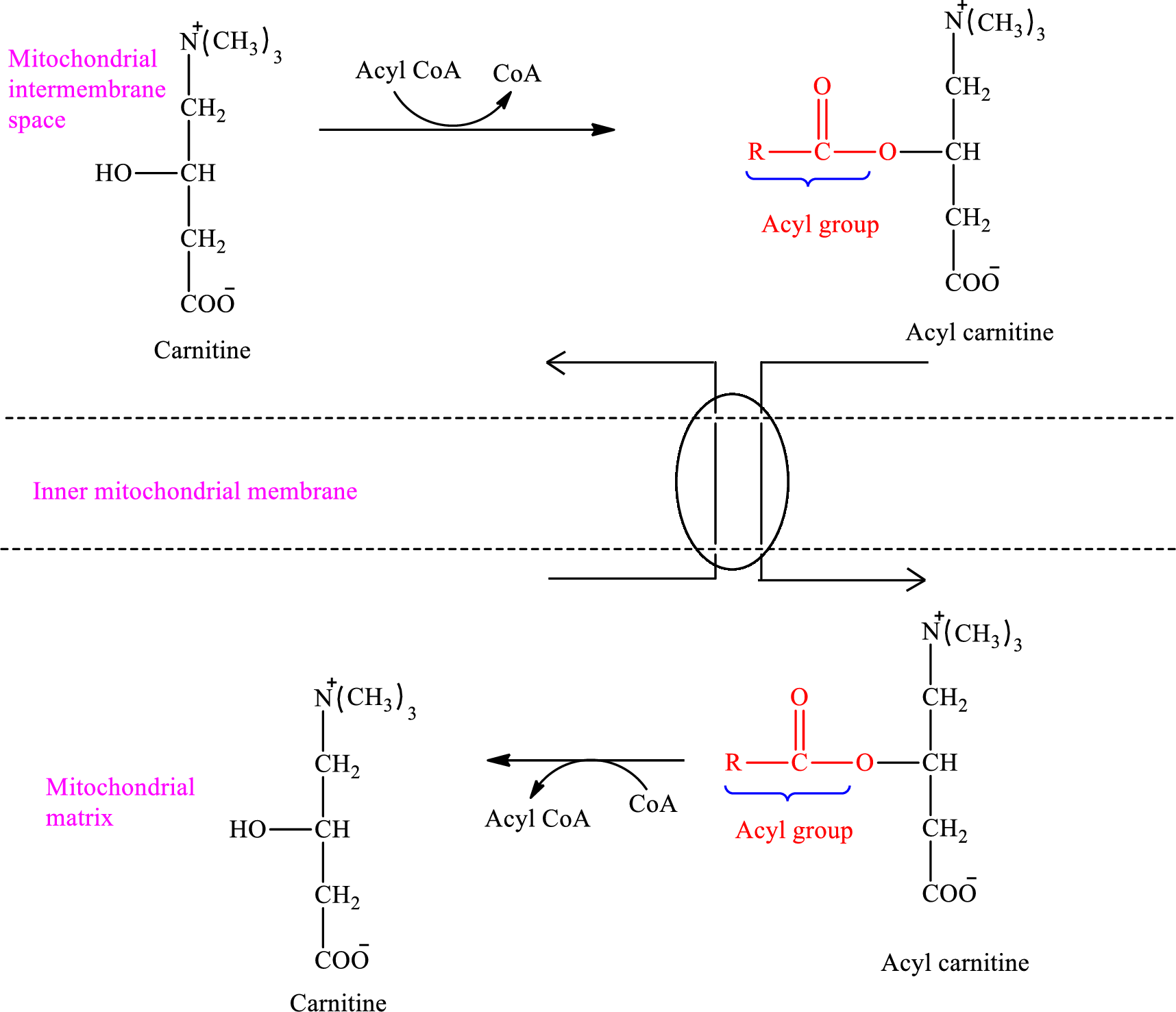
Therefore, free
Want to see more full solutions like this?
Chapter 14 Solutions
Organic And Biological Chemistry
- Calculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forwardPredict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forward
- Experts...can any one help me solve these problems?arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forward
- Which of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forwardWhich element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forwardWhich of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forward
- Which of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forwardConsider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



