
Concept explainers
(a)
Interpretation:
The amino acid stands for the given abbreviation Ser has to be determined.
Concept introduction:
Amino acids are the molecules containing an
General formula for amino acid is given below,
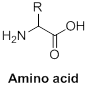
Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
Some amino acids and their corresponding abbreviations are listed below,
(b)
Interpretation:
The amino acid stands for the given abbreviation Thr has to be determined.
Concept introduction:
Amino acids are the molecules containing an amine group, a carboxylic group, and a side-chain that is specific to each amino acid. They are the basic structural building units of protein and other biomolecules.
General formula for amino acid is given below,

Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
Some amino acids and their corresponding abbreviations are listed below,
(c)
Interpretation:
The amino acid stands for the given abbreviation pro has to be determined.
Concept introduction:
Amino acids are the molecules containing an amine group, a carboxylic group, and a side-chain that is specific to each amino acid. They are the basic structural building units of protein and other biomolecules.
General formula for amino acid is given below,

Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
Some amino acids and their corresponding abbreviations are listed below,
(d)
Interpretation:
The amino acid stands for the given abbreviation phe has to be determined.
Concept introduction:
Amino acids are the molecules containing an amine group, a carboxylic group, and a side-chain that is specific to each amino acid. They are the basic structural building units of protein and other biomolecules.
General formula for amino acid is given below,

Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
Some amino acids and their corresponding abbreviations are listed below,
(e)
Interpretation:
The amino acid stands for the given abbreviation Cys has to be determined.
Concept introduction:
Amino acids are the molecules containing an amine group, a carboxylic group, and a side-chain that is specific to each amino acid. They are the basic structural building units of protein and other biomolecules.
General formula for amino acid is given below,

Carbon, hydrogen, oxygen and nitrogen are the key elements in amino acid.
Some amino acids and their corresponding abbreviations are listed below,
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
General Chemistry: Atoms First
- QUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forward
- oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





