
(a)
Interpretation:
The
Concept Introduction:
Ester: One
Esterification reaction: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
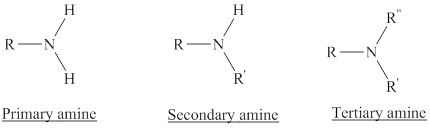
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Aromatic Compounds: Compounds that are planar, conjugate, cyclic and having
(b)
Interpretation:
The products obtained by treating methylphenidate with aqueous base should be drawn.
Concept Introduction:
Carboxylic acid: One
Ester: One
Ester hydrolysis: The splitting of an ester into the component acid and alcohol is known as ester hydrolysis this reaction is reversible and can be catalyzed by acids or bases.
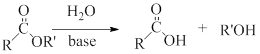
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
General Chemistry: Atoms First
- Predict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forwardulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forwardI apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forward
- Calculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward: At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forwardwhen performing the reaction that involves 2 equivalents of 3-(diethylamino)-phenol and Phthalic anhydride with sulfuric acid and water react to form rhodamine b where the Phthalic anhydride cleaves in acid and how does Excessive Washing (w/ Base) & Subsequent Resonance Structure get affectedarrow_forward
- 3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol Please explain your steps and thought process. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4: Cl2(g) + CHCl3(g) HCl(g) + CC₁(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and 1.6 mol of CCl4. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl2 and CHCl3. ☐ x10 There will be very little HCI and CCl4. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. HCl(g)+CC14(g) 12 Cl2(9)+CHCl3(9) K = 0 ☐ What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 Cl₂(9)+2CHCl3(9) 2 HCl(9)+2CC₁₁(9) K = ✓ 00. 18 Ararrow_forward10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



