
Concept explainers
Interpretation:
The condensed structure and possible isomers should be drawn and identified for the given molecular model structure.
Concept Introduction:
Condensed Structure: It is the one, where the symbols of atoms are listed in order as they appear in the molecules structure with bond dashes omitted or limited.
- 1. A molecular formula explains only the number of atoms of each element in a molecule of the compound.
- 2. Condensed structure shows all atoms, but it omits the vertical bonds and most or all of the vertical bonds and most or all of the horizontal single bonds.
Isomers: The molecules with same molecular formula but differ in the way in which the atoms are arranged is known as isomers.
In other words compounds have the same molecular formula but have different structural formula and different names.
Answer to Problem 23.33CP
Condensed structure of given molecular model is,
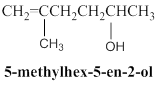
Explanation of Solution
Given molecular model presence of seven carbon atoms and 14 hydrogen atoms and all hydrogen atoms horizontally connected to respective carbon atoms, so given molecular model is correctly matched for a 5-methylhex-5-ene-2-ol molecule and condensed structure is shown below.
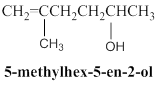
Determination of possible isomers:
Above the condensed structure was found to have two possible isomers, the possible isomers structure are shown below,
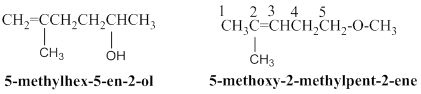
5-methyl hex-5-en-2-ol:
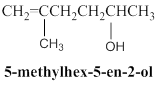
The above structure, 11 hydrogen atoms are horizontally connected to respective carbon atoms, one methyl groups is connected in branched position and another one hydroxyl group connected in
5-methoxy-2-methylpent-2-ene:
Given molecular model structure was founded to have another possible isomers,
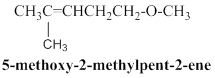
In second molecule 11 hydrogen atoms are horizontally connected to respective carbon atoms, and one methyl group is connected in branched position, than another one methoxy group connected in
For the given molecular model, the condensed structure was drawn and possible isomers were determined.
Want to see more full solutions like this?
Chapter 23 Solutions
General Chemistry: Atoms First
- 13 How many signals would you expect to see in the Check O signal(s) X § 'C NMR spectrum for the following compound? © 2025 McGraw Hillarrow_forward13 Consider the "C NMR spectrum below. 140 120 100 80 60 40 20 20 PPM 0 The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer. Check ✓ G Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below. How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)? Check ×arrow_forward
- 1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forwardwrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forward
- Study this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forward
- an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forwardAre there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

