
Concept explainers
(a)
Interpretation:
The number of nitrogen atom present in unsubstituted amide has to be given.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are
One of the
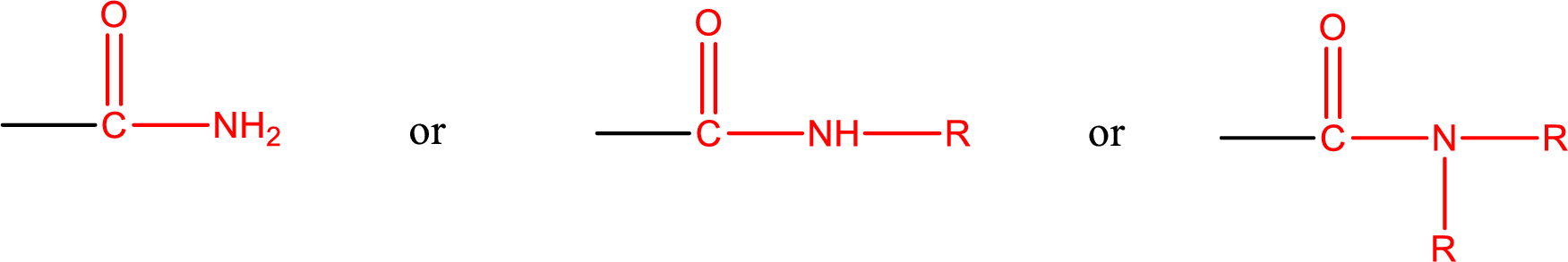
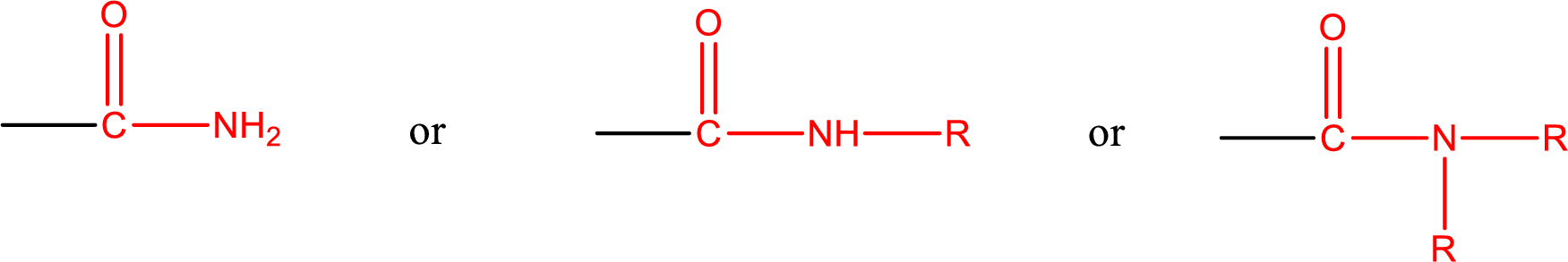
Amides are also classified as primary, secondary, and tertiary amide.
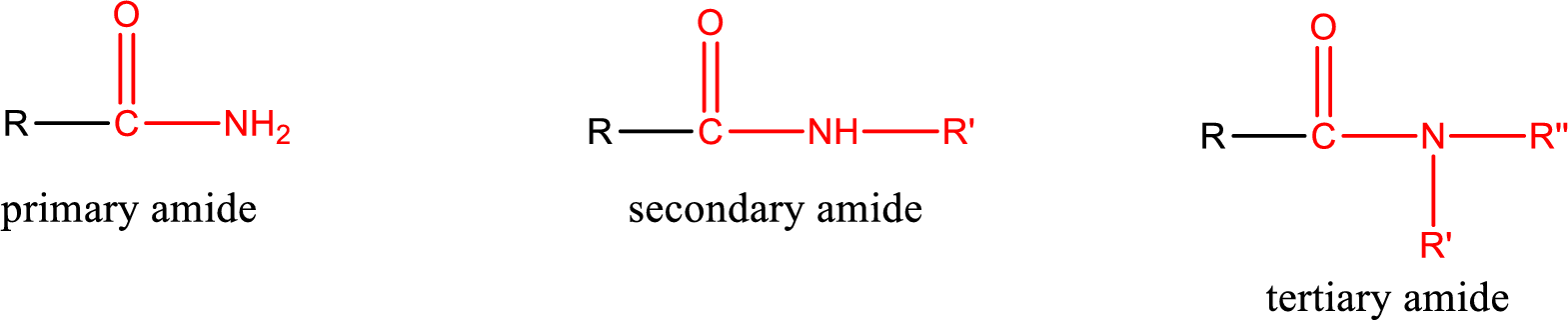
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
(b)
Interpretation:
The number of nitrogen atoms present in disubstituted amide has to be given.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are amines, amides.
One of the carboxylic acid derivative is amide. In this the carboxyl
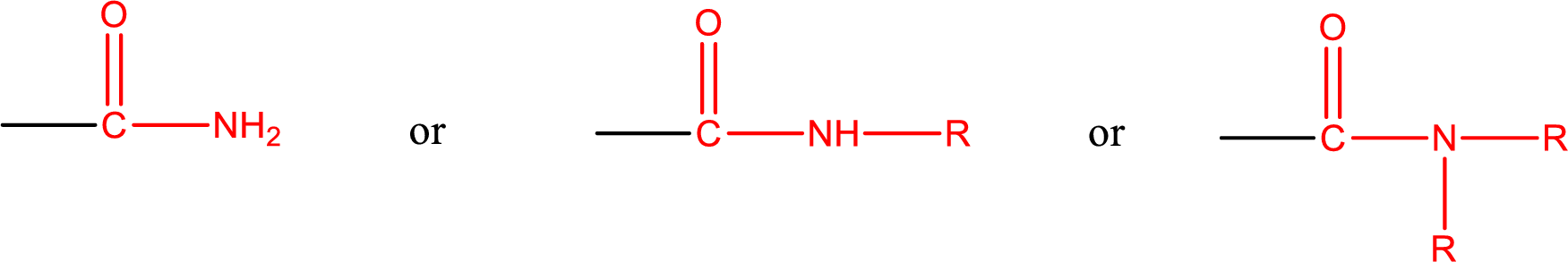
Amides are also classified as primary, secondary, and tertiary amide.
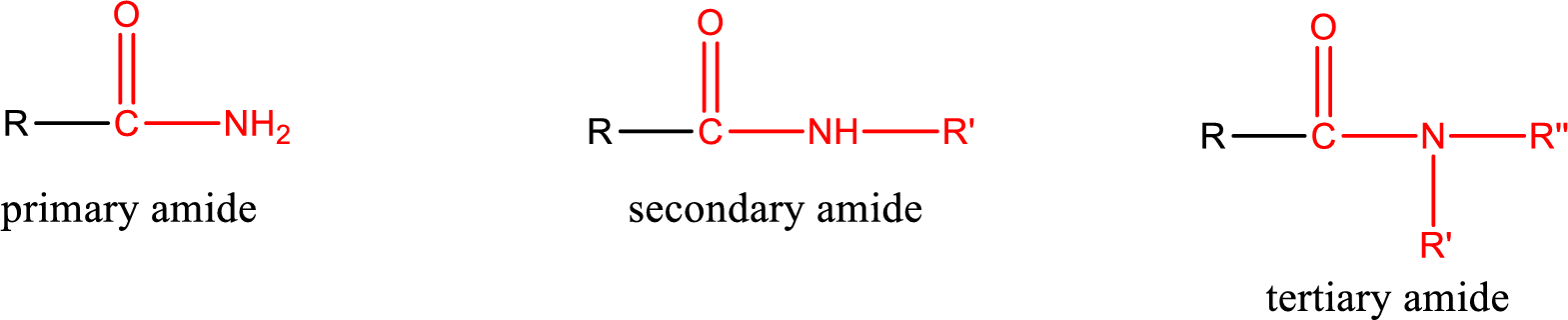
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
(c)
Interpretation:
The number of nitrogen atoms present in secondary amide has to be given.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Organic compounds contain heteroatom also. Some of them are nitrogen, sulfur, oxygen etc. Nitrogen containing organic compounds are of two important types and they are amines, amides.
One of the carboxylic acid derivative is amide. In this the carboxyl
Amides are also classified as primary, secondary, and tertiary amide.
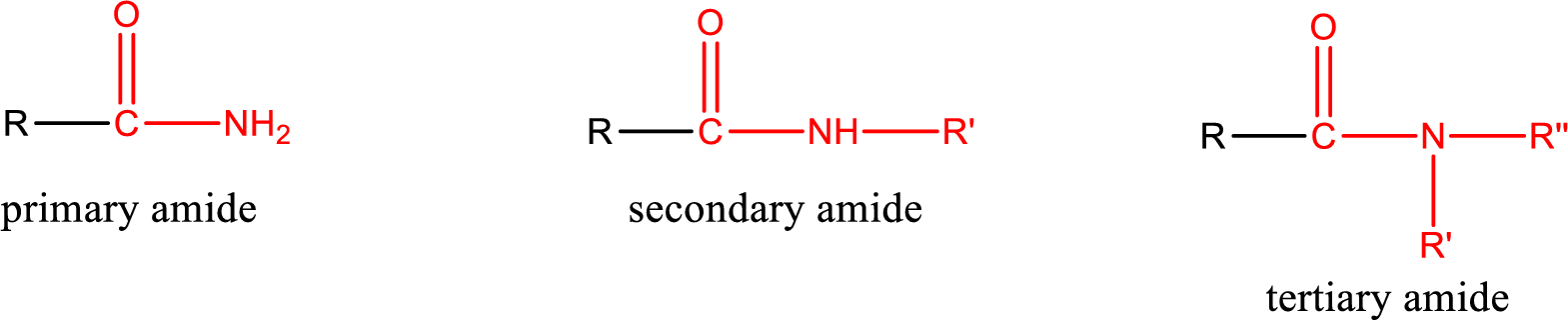
Primary amide is the one that has a nitrogen atom that is bonded to two hydrogen atoms. Primary amides are also known as unsubstituted amides.
Secondary amide is the one that has a nitrogen atom that is bonded to one hydrogen atom and one alkyl (or aryl) group. Secondary amides are also known as monosubstituted amides.
Tertiary amide is the one that has a nitrogen atom that is bonded to two alkyl (or aryl) groups. Tertiary amides are also known as disubstituted amides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





