
Concept explainers
(a)
Interpretation:
“Parent” amine can be regenerated from the given salt or not has to be indicated.
Concept Introduction:
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with
Neutralization reaction is the one that takes place between an acid and a base to give salt as product. As
When a strong base is added to the amine salt, the parent amine can be obtained. This is a reverse reaction of the amine salt formation reaction. These reactions can be represented as shown below,
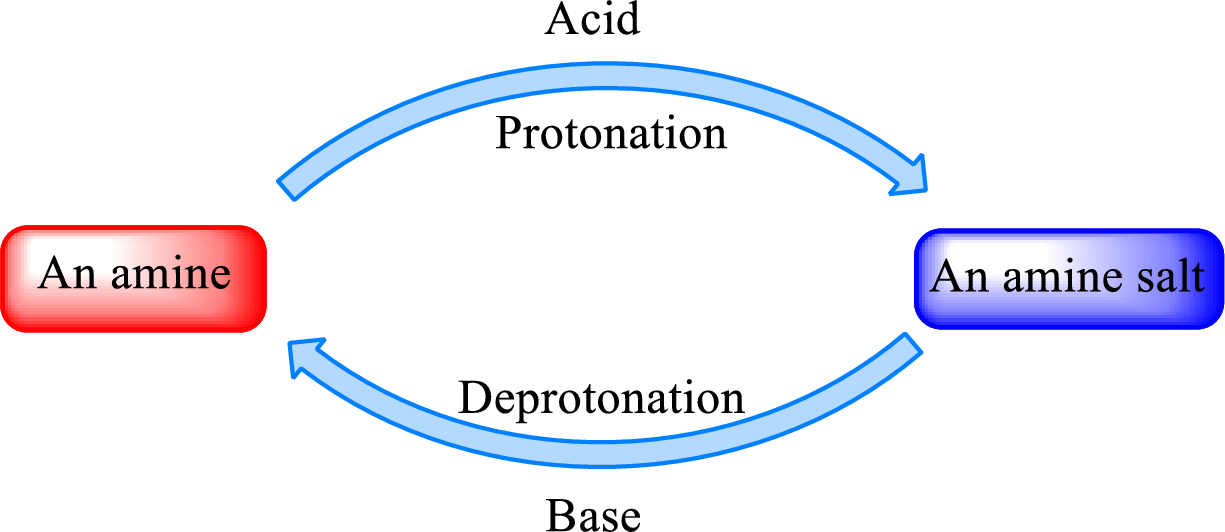
Quaternary ammonium salt does not give the “parent” amine when treated with a strong base as there is no possibility of deprotonation to take place.
(b)
Interpretation:
“Parent” amine can be regenerated from the given salt or not has to be indicated.
Concept Introduction:
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
Neutralization reaction is the one that takes place between an acid and a base to give salt as product. As amines are bases due to the amino group in it, the reaction with inorganic acid or carboxylic acid gives salt as product. The salt formed is an amine salt. Proton is donated from the acid to the nitrogen atom which acts as a proton acceptor. In simple words, it can be said that in an amine‑acid reaction, the acid loses a hydrogen ion and amine gains a hydrogen ion.
When a strong base is added to the amine salt, the parent amine can be obtained. This is a reverse reaction of the amine salt formation reaction. These reactions can be represented as shown below,
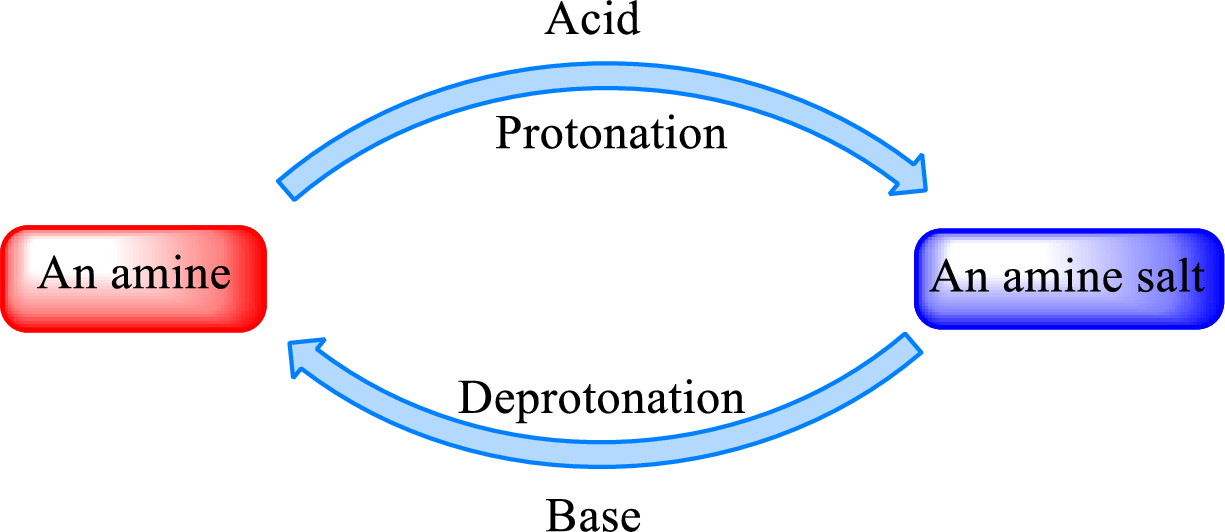
Quaternary ammonium salt does not give the “parent” amine when treated with a strong base as there is no possibility of deprotonation to take place.
(c)
Interpretation:
“Parent” amine can be regenerated from the given salt or not has to be indicated.
Concept Introduction:
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
Neutralization reaction is the one that takes place between an acid and a base to give salt as product. As amines are bases due to the amino group in it, the reaction with inorganic acid or carboxylic acid gives salt as product. The salt formed is an amine salt. Proton is donated from the acid to the nitrogen atom which acts as a proton acceptor. In simple words, it can be said that in an amine‑acid reaction, the acid loses a hydrogen ion and amine gains a hydrogen ion.
When a strong base is added to the amine salt, the parent amine can be obtained. This is a reverse reaction of the amine salt formation reaction. These reactions can be represented as shown below,
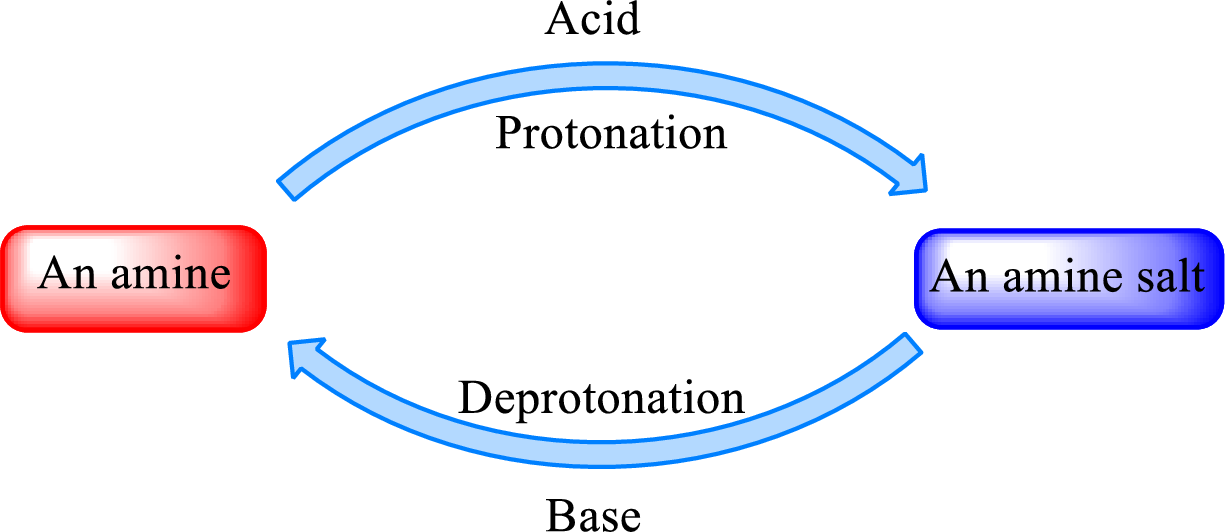
Quaternary ammonium salt does not give the “parent” amine when treated with a strong base as there is no possibility of deprotonation to take place.
(d)
Interpretation:
“Parent” amine can be regenerated from the given salt or not has to be indicated.
Concept Introduction:
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
Neutralization reaction is the one that takes place between an acid and a base to give salt as product. As amines are bases due to the amino group in it, the reaction with inorganic acid or carboxylic acid gives salt as product. The salt formed is an amine salt. Proton is donated from the acid to the nitrogen atom which acts as a proton acceptor. In simple words, it can be said that in an amine‑acid reaction, the acid loses a hydrogen ion and amine gains a hydrogen ion.
When a strong base is added to the amine salt, the parent amine can be obtained. This is a reverse reaction of the amine salt formation reaction. These reactions can be represented as shown below,
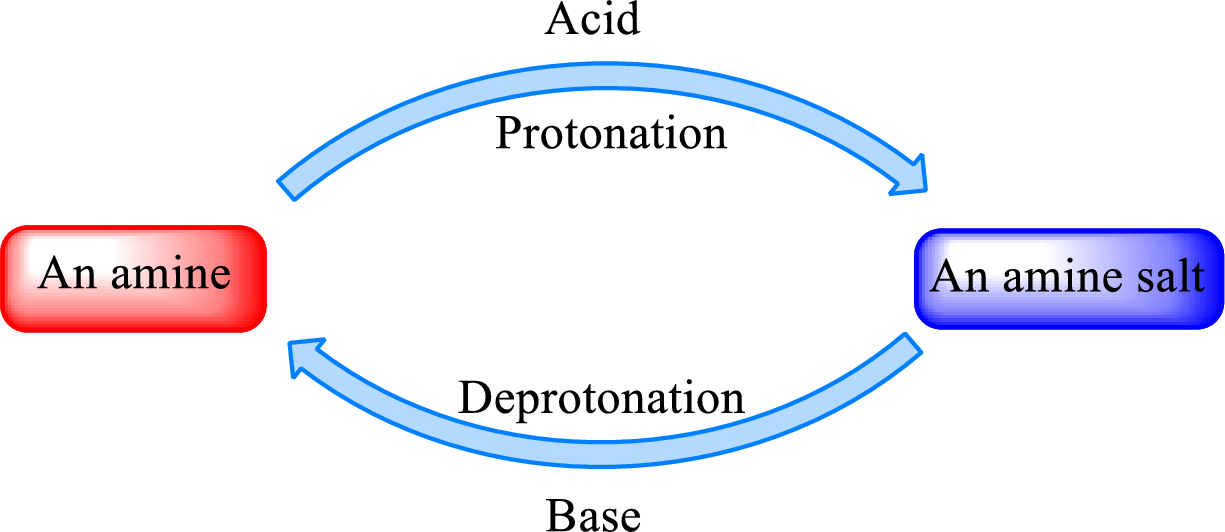
Quaternary ammonium salt does not give the “parent” amine when treated with a strong base as there is no possibility of deprotonation to take place.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Which Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forwardWhich element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forward
- Which of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forwardWhich of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forwardConsider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardConsider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forwardWhat is the missing reactant in this organic reaction? OH H + R Δ CH3-CH2-CH-CH3 O CH3 CH3-CH2-C-O-CH-CH2-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answe box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C O2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cerarrow_forward
- Predict the product of this organic reaction: CH3 NH2 Δ CH3-CH-CH3 + HO-C-CH2-N-CH3 P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. Xarrow_forwardIn the scope of the SCH4U course, please thoroughly go through the second questionarrow_forwardPlease help me solve these two problems. Thank you in advance.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




