
(a)
Interpretation:
Dopamine and serotonin have same tryptamine core or not has to be indicated as true or false.
Concept Introduction:
Generally
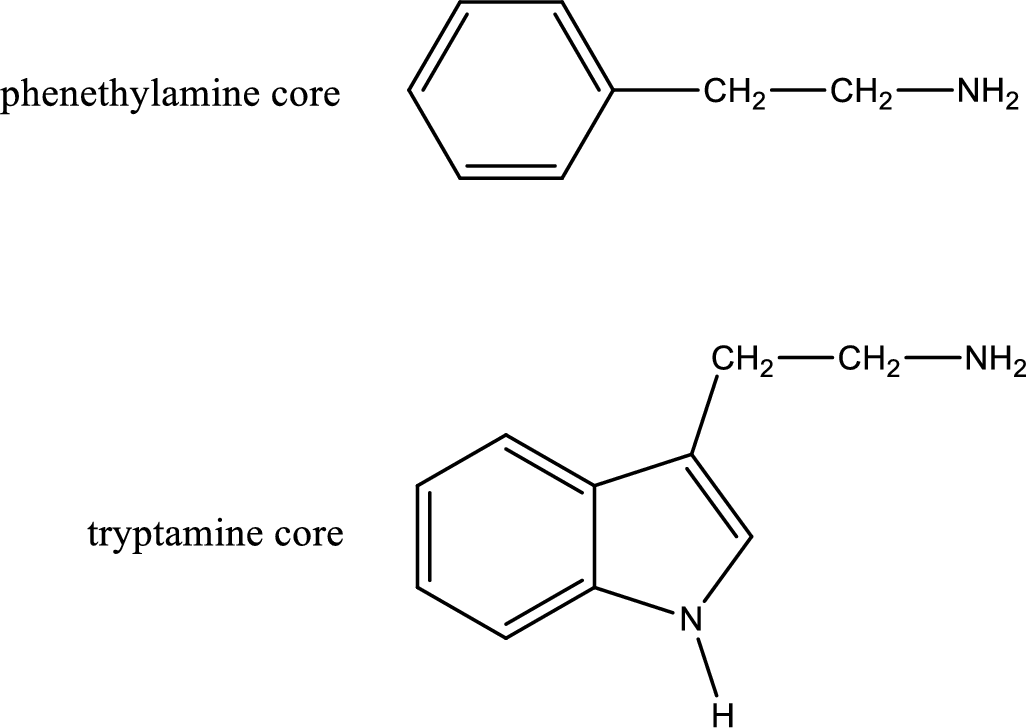
There are three types of effects that an amine can exert. They are neurotransmitters, central nervous system stimulants and decongestants. Neurotransmitters are the substances that are present in human body that help in passing impulse of nerves from one cell to another. Central nervous stimulants are the substances that help in speeding up of physical and mental processes. Decongestant is a substance that is used to relieve nasal congestion.
(b)
Interpretation:
Parkinson’s disease is due to the deficiency of dopamine has to be indicated as true or false.
Concept Introduction:
Generally amines are toxic in nature. If a compound contains only amine as its functional group, they will be often toxic. Due to this, in biological systems they are not prevalent. If the same compound contains more than one functional group apart from amine means it can be physiologically active. Important “core” structures that are frequently encountered in polyfunctional amines that have biological effects are tryptamine core and phenethylamine core.
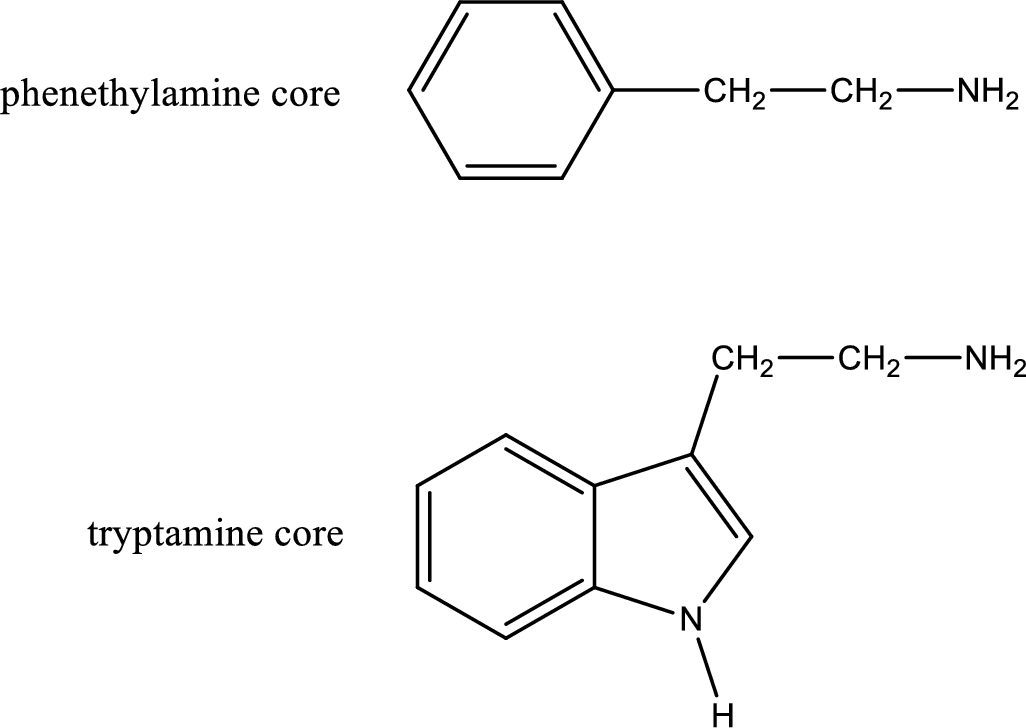
There are three types of effects that an amine can exert. They are neurotransmitters, central nervous system stimulants and decongestants. Neurotransmitters are the substances that are present in human body that help in passing impulse of nerves from one cell to another. Central nervous stimulants are the substances that help in speeding up of physical and mental processes. Decongestant is a substance that is used to relieve nasal congestion.
(c)
Interpretation:
Epinephrine and norepinephrine differs by a methyl group only has to be indicated true or false.
Concept Introduction:
Generally amines are toxic in nature. If a compound contains only amine as its functional group, they will be often toxic. Due to this, in biological systems they are not prevalent. If the same compound contains more than one functional group apart from amine means it can be physiologically active. Important “core” structures that are frequently encountered in polyfunctional amines that have biological effects are tryptamine core and phenethylamine core.
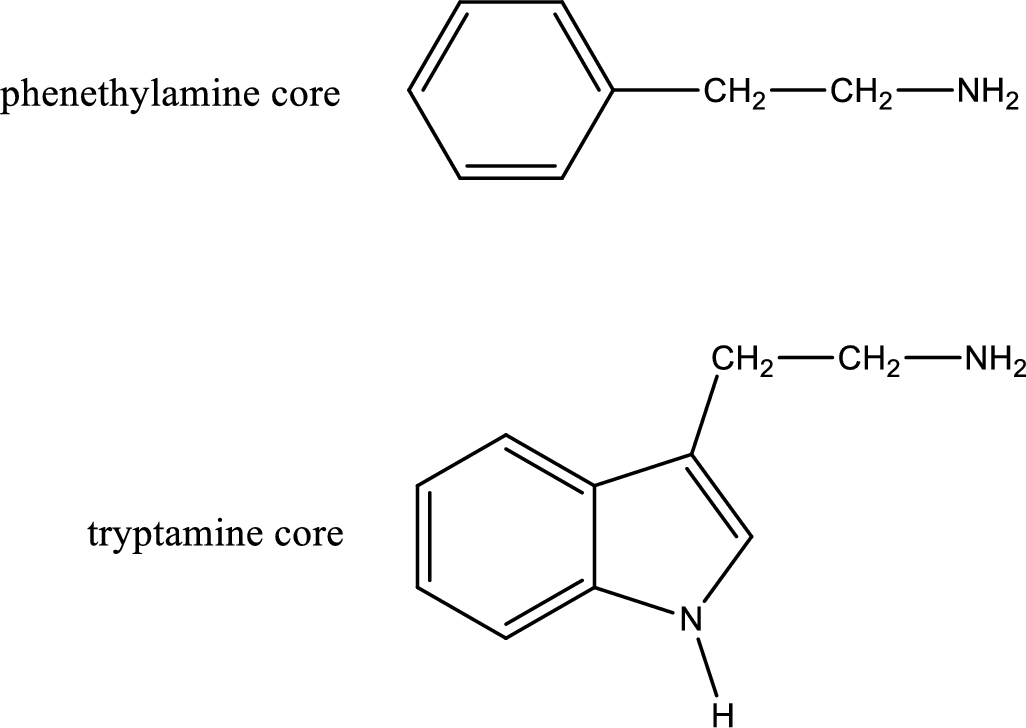
There are three types of effects that an amine can exert. They are neurotransmitters, central nervous system stimulants and decongestants. Neurotransmitters are the substances that are present in human body that help in passing impulse of nerves from one cell to another. Central nervous stimulants are the substances that help in speeding up of physical and mental processes. Decongestant is a substance that is used to relieve nasal congestion.
(d)
Interpretation:
Epinephrine and adrenaline are the names of same compound has to be indicated as true or false.
Concept Introduction:
Generally amines are toxic in nature. If a compound contains only amine as its functional group, they will be often toxic. Due to this, in biological systems they are not prevalent. If the same compound contains more than one functional group apart from amine means it can be physiologically active. Important “core” structures that are frequently encountered in polyfunctional amines that have biological effects are tryptamine core and phenethylamine core.
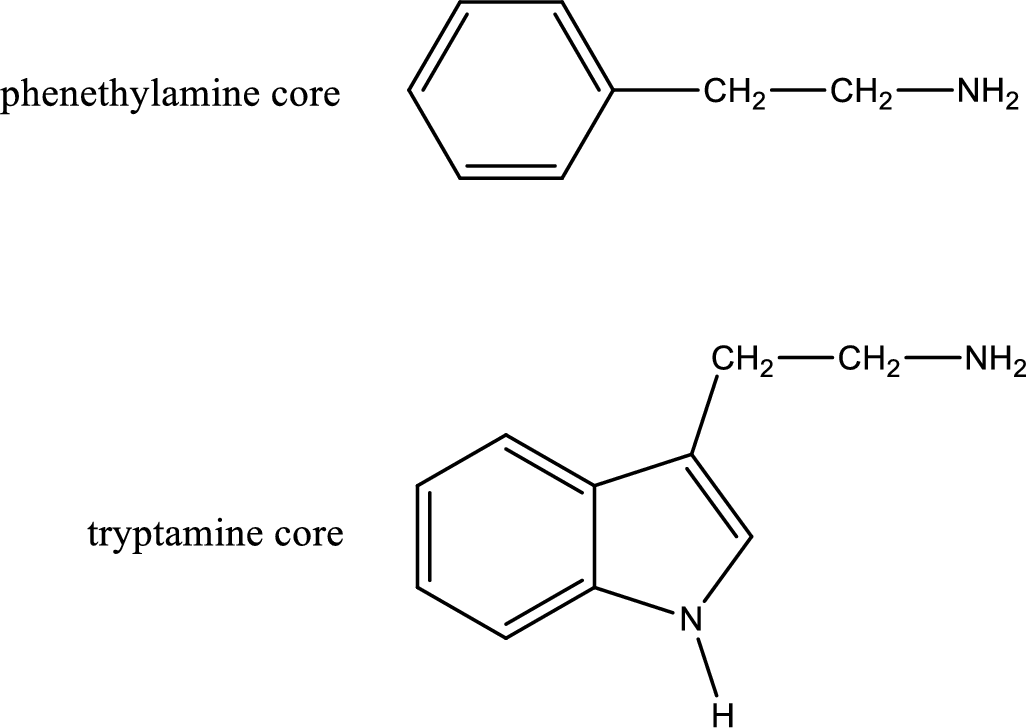
There are three types of effects that an amine can exert. They are neurotransmitters, central nervous system stimulants and decongestants. Neurotransmitters are the substances that are present in human body that help in passing impulse of nerves from one cell to another. Central nervous stimulants are the substances that help in speeding up of physical and mental processes. Decongestant is a substance that is used to relieve nasal congestion.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- julietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardThe electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forward
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





