
Concept explainers
(a)
Interpretation:
The structure of nitrogen-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of
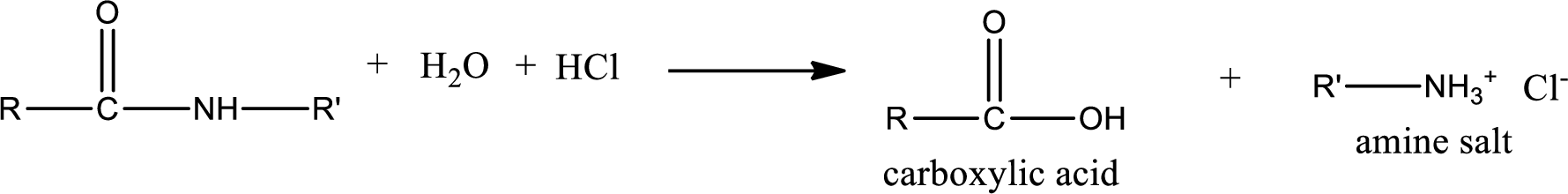
Acidic hydrolysis of amides gives the product as carboxylic acid and
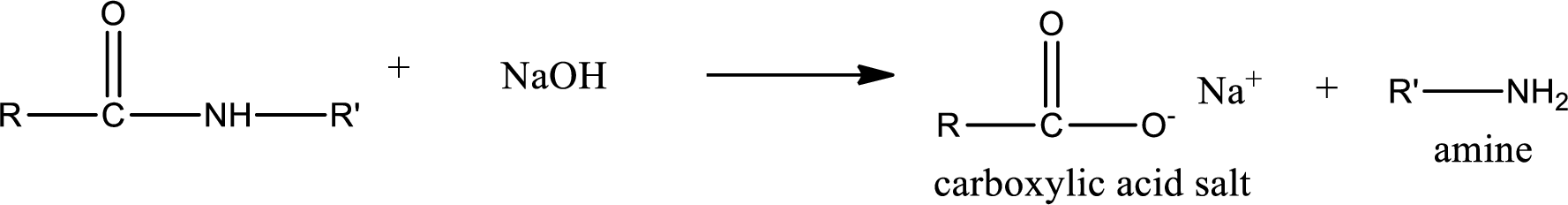
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
(b)
Interpretation:
The structure of nitrogen-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
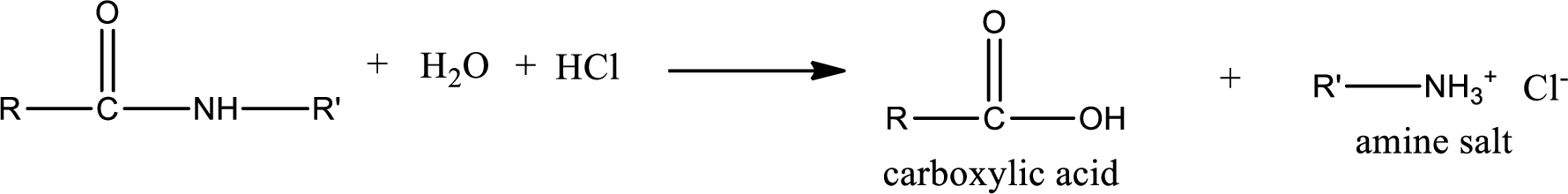
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
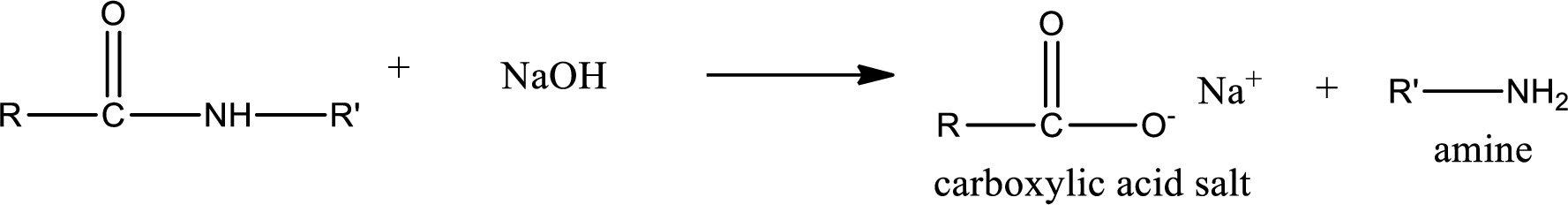
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
(c)
Interpretation:
The structure of nitrogen-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
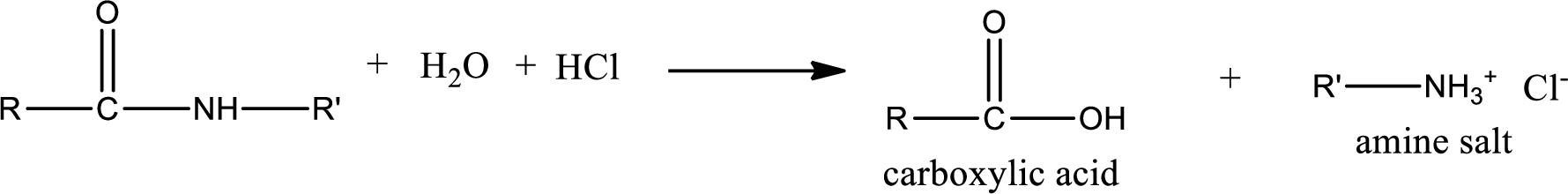
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
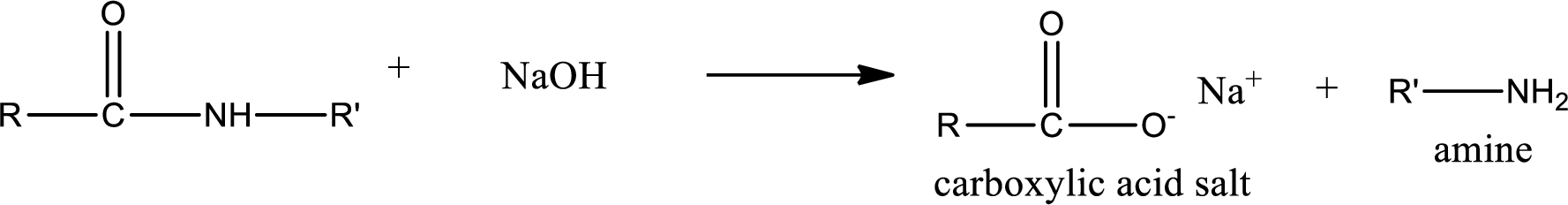
(d)
Interpretation:
The structure of nitrogen-containing compound that is obtained when the given amide undergoes acidic hydrolysis has to be drawn.
Concept Introduction:
Amides are derivatives of carboxylic acid. Amides are not much reactive as of carboxylic acids. They are also stable relatively in aqueous solution. But under strenuous conditions amides undergo hydrolysis. The conditions are presence of acid, base or enzymes.
Acidic hydrolysis of amides gives the product as carboxylic acid and amine salt. Amine salt is obtained because in acidic conditions the amine is converted into amine salt.
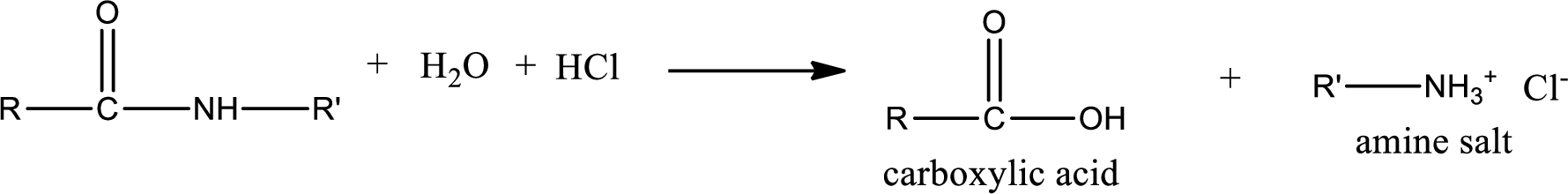
Basic hydrolysis of amides gives the product as carboxylic acid salt and amine. Carboxylic acid salt is obtained because in basic conditions the carboxylic acid is converted into carboxylic acid salt.
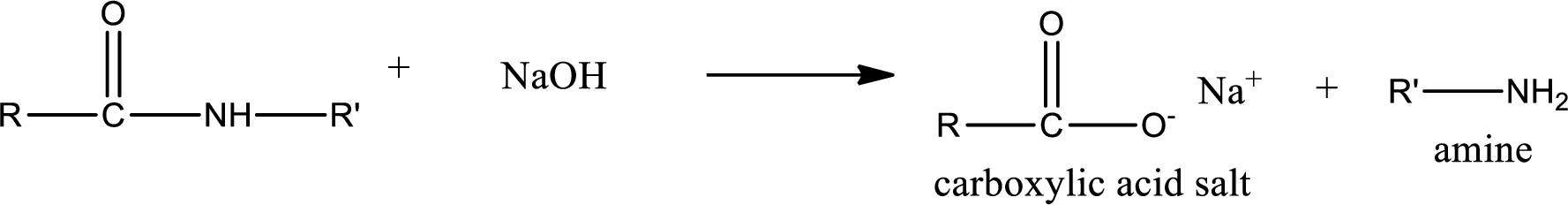
Trending nowThis is a popular solution!

Chapter 6 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

