
Indicate whether each of the following aspects of triacylglycerol digestion is associated with the (1) mouth, (2) stomach, (3) small intestine, (4) intestinal cells, or (5) bloodstream.
- a. Chyme is produced.
- b. Gastric lipases are active.
- c. Initial production of monoacylglycerols occurs.
- d. Fatty acid micelles are formed.
(a)
Interpretation: To identify whether the chyme is produced in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: In the stomach, the muscle walls of the stomach churn the food and other complex molecules into smaller particles. The churning action breaks up the lipid molecules into small droplets/globules which remains suspended on the other smaller components of the ingested food. This results in the formation of a semi-liquid material comprising of small lipid droplets, partly digested food and gastric secretions called chyme. The chyme, thereafter, resumes its journey to the small intestine.
Answer to Problem 14.1EP
Chyme production occurs in the stomach.
Explanation of Solution
Chyme is a semi-liquid material composed of small lipid droplets, partly digested food and gastric secretions. Since the initial breakdown of lipid molecules and activity of the gastric lipases begins in the stomach, hence the formation of chyme occurs in the stomach.
(b)
Interpretation: To identify whether the gastric lipase is active in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Gastric lipase is an acidic lipase secreted by the gastric chief cells in the fundic mucosa in the stomach. The function of the enzyme is to break down lipid molecules. Gastric lipases are enzymes which break down lipids.
Answer to Problem 14.1EP
Gastric lipases are active in the stomach.
Explanation of Solution
Gastric lipases are present in the stomach and help in the initial hydrolysis of the triacylglycerol molecules. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). Around 10% of the triacylglycerols are hydrolyzed in the stomach by the action of gastric lipases. Hence, gastric lipases are active in the stomach.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
(c)
Interpretation: To identify whether the initial production of monoacylglycerol occurs in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: The initial lipid digestion begins in the stomach in the presence of gastric lipases. Gastric lipases are enzymes which break down lipids. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. Here the digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
Answer to Problem 14.1EP
The initial production of monoacylglycerol occurs in the stomach.
Explanation of Solution
In the stomach, around 10% of the triacylglycerol is hydrolyzed into monoacylglycerol and free fatty acids under the activity of gastric lipases. The remaining hydrolysis occurs in the small intestine. Hence, the initial production of monoacylglycerol occurs in the stomach.
(d)
Interpretation: To identify whether the fatty acid micelles are formed in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
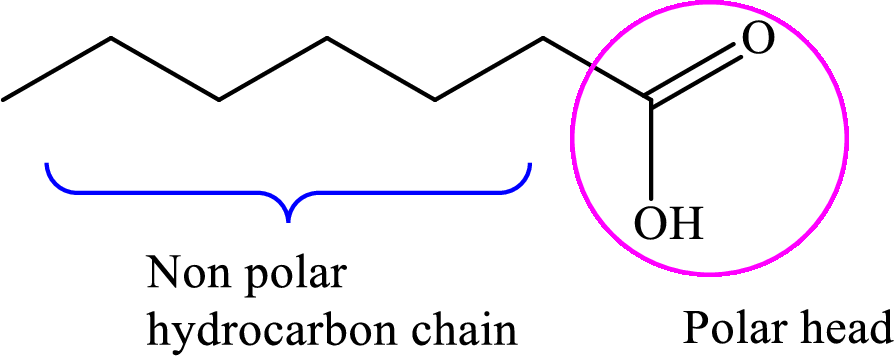
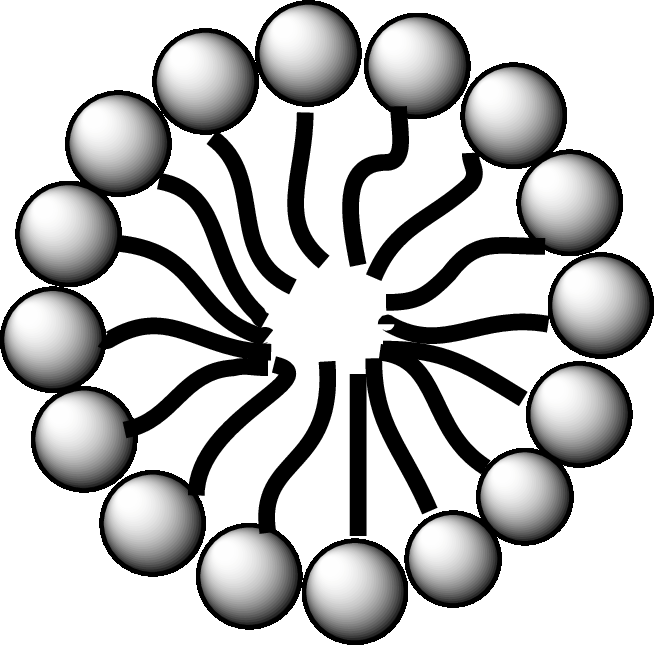
Concept introduction: Fatty acid is a carboxylic acid that contains a long hydrophobic hydrocarbon chain and a polar carboxyl group. The fatty acids are aligned such that the hydrophobic hydrocarbon chain is directed inwards away from the polar environment and the polar carboxyl group head is directed outwards. The aggregate thus formed is called micelle.
A general representation of fatty acid is,
A diagrammatic representation of micelle is as follows:
Answer to Problem 14.1EP
Fatty acid micelles are formed in the small intestine.
Explanation of Solution
A fatty acid micelle is a spherical droplet which is primarily composed of free fatty acids, monoacylglycerols, and bile.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
In the small intestine, the monoacylglycerols and free fatty acids along with bile and pancreatic lipases, form micelles. Micelles are later absorbed by the cell membranes of the intestinal cells.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic And Biological Chemistry
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
General, Organic, and Biological Chemistry - 4th edition
Human Anatomy & Physiology (2nd Edition)
Anatomy & Physiology (6th Edition)
Biology: Concepts and Investigations
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





