
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 10E
Interpretation Introduction
Interpretation: The rearrangement of below carbocations should be drawn bu curved arrows.
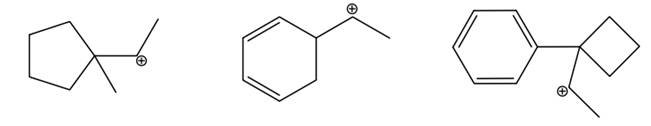
Concept introduction: Carbocation is a general term employed for a postively charged carbon irrespective of valency of carbon. In carbocation, carbon is bonded to 3 atoms or groups and has only sextet of electrons so it behaves as an electron-deficient species. It is
The order of relative stability of various possible carbocation species is as follows:
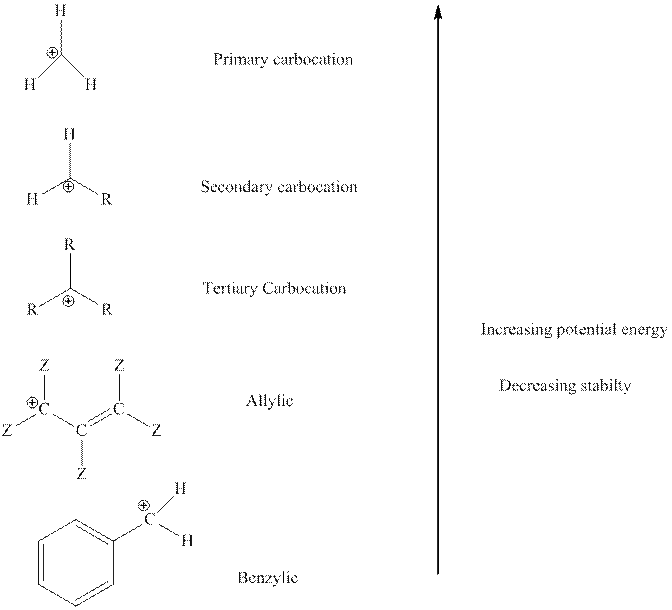
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
First image: I have to explain why the molecule C is never formed in those conditions.
Second image: I have to propose a synthesis for the lactone A
First image: I have to explain why the molecule C is never formed in these conditions
Second image: I have to propose a synthesis for the lactone A
Help fix my arrows please
Chapter 8 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 8 - Prob. 1CTQCh. 8 - Prob. 2CTQCh. 8 - Prob. 3CTQCh. 8 - Prob. 4CTQCh. 8 - Prob. 5CTQCh. 8 - Prob. 6CTQCh. 8 - Prob. 7CTQCh. 8 - Prob. 8CTQCh. 8 - Prob. 9CTQCh. 8 - Prob. 10CTQ
Ch. 8 - Draw the products that result from the electron...Ch. 8 - Prob. 12CTQCh. 8 - Draw the products that would result if the arrow...Ch. 8 - Prob. 14CTQCh. 8 - What information (if any) from the following...Ch. 8 - Prob. 16CTQCh. 8 - Prob. 17CTQCh. 8 - The reactants, intermediates, final products, and...Ch. 8 - Prob. 19CTQCh. 8 - Prob. 20CTQCh. 8 - Prob. 21CTQCh. 8 - Prob. 22CTQCh. 8 - Explain how you can tell from the energy diagram...Ch. 8 - Explain why the following mechanism for hydration...Ch. 8 - Prob. 25CTQCh. 8 - Prob. 26CTQCh. 8 - Prob. 27CTQCh. 8 - Prob. 28CTQCh. 8 - Prob. 29CTQCh. 8 - Prob. 30CTQCh. 8 - Prob. 31CTQCh. 8 - The hydration above is one of a family of...Ch. 8 - Prob. 33CTQCh. 8 - Which statement is false? a. A mechanistic step...Ch. 8 - Prob. 35CTQCh. 8 - Prob. 36CTQCh. 8 - Prob. 37CTQCh. 8 - Draw the complete mechanism including the...Ch. 8 - Prob. 2ECh. 8 - Explain why ethene does not react with HX ( X=Cl ,...Ch. 8 - Draw the complete mechanism of each pair of...Ch. 8 - Prob. 5ECh. 8 - Prob. 6ECh. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - Prob. 10ECh. 8 - Prob. 11ECh. 8 - Prob. 12ECh. 8 - Prob. 15ECh. 8 - A student proposes the following reaction...Ch. 8 - Prob. 17ECh. 8 - Prob. 18ECh. 8 - Prob. 19E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning