
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.12, Problem 2QQ
Interpretation Introduction
Interpretation:
The correct IUPAC name for the ester given has to be chosen from the given options.
Concept Introduction:
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
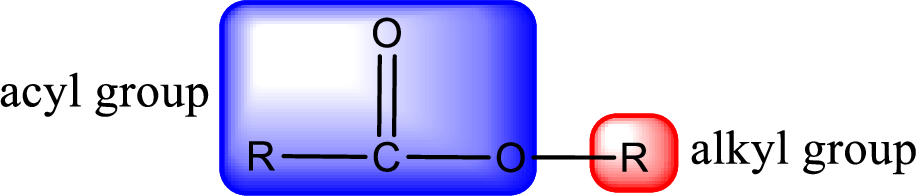
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 5 Solutions
Organic And Biological Chemistry
Ch. 5.1 - Prob. 1QQCh. 5.1 - Prob. 2QQCh. 5.1 - Prob. 3QQCh. 5.1 - Prob. 4QQCh. 5.1 - Prob. 5QQCh. 5.2 - Prob. 1QQCh. 5.2 - Prob. 2QQCh. 5.2 - Prob. 3QQCh. 5.2 - Prob. 4QQCh. 5.2 - Prob. 5QQ
Ch. 5.3 - Prob. 1QQCh. 5.3 - Prob. 2QQCh. 5.3 - Prob. 3QQCh. 5.4 - Prob. 1QQCh. 5.4 - Prob. 2QQCh. 5.4 - Prob. 3QQCh. 5.5 - Prob. 1QQCh. 5.5 - Prob. 2QQCh. 5.5 - Prob. 3QQCh. 5.6 - Prob. 1QQCh. 5.6 - Prob. 2QQCh. 5.7 - Which of the following statements about acid...Ch. 5.7 - Prob. 2QQCh. 5.7 - Prob. 3QQCh. 5.8 - Prob. 1QQCh. 5.8 - Prob. 2QQCh. 5.8 - Prob. 3QQCh. 5.8 - Prob. 4QQCh. 5.9 - Prob. 1QQCh. 5.9 - Prob. 2QQCh. 5.10 - Prob. 1QQCh. 5.10 - Prob. 2QQCh. 5.11 - Prob. 1QQCh. 5.11 - Prob. 2QQCh. 5.11 - Prob. 3QQCh. 5.12 - Prob. 1QQCh. 5.12 - Prob. 2QQCh. 5.12 - Prob. 3QQCh. 5.12 - Prob. 4QQCh. 5.13 - Prob. 1QQCh. 5.13 - Prob. 2QQCh. 5.14 - Prob. 1QQCh. 5.14 - Prob. 2QQCh. 5.14 - Prob. 3QQCh. 5.15 - Prob. 1QQCh. 5.15 - Prob. 2QQCh. 5.15 - Prob. 3QQCh. 5.16 - Prob. 1QQCh. 5.16 - Prob. 2QQCh. 5.16 - Prob. 3QQCh. 5.17 - Prob. 1QQCh. 5.17 - Prob. 2QQCh. 5.18 - Prob. 1QQCh. 5.18 - Prob. 2QQCh. 5.18 - Prob. 3QQCh. 5.19 - Prob. 1QQCh. 5.19 - Prob. 2QQCh. 5.19 - Prob. 3QQCh. 5.19 - Prob. 4QQCh. 5.20 - Prob. 1QQCh. 5.20 - Prob. 2QQCh. 5.20 - Prob. 3QQCh. 5.20 - Prob. 4QQCh. 5 - Prob. 5.1EPCh. 5 - Prob. 5.2EPCh. 5 - Prob. 5.3EPCh. 5 - Indicate whether or not each of the compounds in...Ch. 5 - Prob. 5.5EPCh. 5 - Prob. 5.6EPCh. 5 - Prob. 5.7EPCh. 5 - Prob. 5.8EPCh. 5 - Prob. 5.9EPCh. 5 - Prob. 5.10EPCh. 5 - Prob. 5.11EPCh. 5 - Prob. 5.12EPCh. 5 - Prob. 5.13EPCh. 5 - Prob. 5.14EPCh. 5 - Prob. 5.15EPCh. 5 - Assign an IUPAC name to each of the following...Ch. 5 - Prob. 5.17EPCh. 5 - Prob. 5.18EPCh. 5 - Prob. 5.19EPCh. 5 - Prob. 5.20EPCh. 5 - Prob. 5.21EPCh. 5 - Prob. 5.22EPCh. 5 - Prob. 5.23EPCh. 5 - Prob. 5.24EPCh. 5 - Prob. 5.25EPCh. 5 - Prob. 5.26EPCh. 5 - Prob. 5.27EPCh. 5 - Prob. 5.28EPCh. 5 - Prob. 5.29EPCh. 5 - Prob. 5.30EPCh. 5 - Prob. 5.31EPCh. 5 - Prob. 5.32EPCh. 5 - Prob. 5.33EPCh. 5 - Prob. 5.34EPCh. 5 - Prob. 5.35EPCh. 5 - Prob. 5.36EPCh. 5 - Prob. 5.37EPCh. 5 - Prob. 5.38EPCh. 5 - Prob. 5.39EPCh. 5 - Prob. 5.40EPCh. 5 - Determine the maximum number of hydrogen bonds...Ch. 5 - Prob. 5.42EPCh. 5 - Prob. 5.43EPCh. 5 - Prob. 5.44EPCh. 5 - Prob. 5.45EPCh. 5 - Prob. 5.46EPCh. 5 - Prob. 5.47EPCh. 5 - Prob. 5.48EPCh. 5 - Prob. 5.49EPCh. 5 - Prob. 5.50EPCh. 5 - Prob. 5.51EPCh. 5 - Prob. 5.52EPCh. 5 - Prob. 5.53EPCh. 5 - Prob. 5.54EPCh. 5 - Draw structural formulas for the following...Ch. 5 - Prob. 5.56EPCh. 5 - Give the IUPAC name for each of the following...Ch. 5 - Give the IUPAC name for each of the following...Ch. 5 - Prob. 5.59EPCh. 5 - Prob. 5.60EPCh. 5 - Prob. 5.61EPCh. 5 - Prob. 5.62EPCh. 5 - Prob. 5.63EPCh. 5 - Prob. 5.64EPCh. 5 - Prob. 5.65EPCh. 5 - Which three carboxylic acids have salts that are...Ch. 5 - Prob. 5.67EPCh. 5 - Which carboxylic acid has salts that are used to...Ch. 5 - Prob. 5.69EPCh. 5 - Prob. 5.70EPCh. 5 - Prob. 5.71EPCh. 5 - Prob. 5.72EPCh. 5 - Prob. 5.73EPCh. 5 - Prob. 5.74EPCh. 5 - Prob. 5.75EPCh. 5 - Prob. 5.76EPCh. 5 - Prob. 5.77EPCh. 5 - Prob. 5.78EPCh. 5 - Prob. 5.79EPCh. 5 - Prob. 5.80EPCh. 5 - Prob. 5.81EPCh. 5 - Prob. 5.82EPCh. 5 - Prob. 5.83EPCh. 5 - Prob. 5.84EPCh. 5 - Prob. 5.85EPCh. 5 - Prob. 5.86EPCh. 5 - Prob. 5.87EPCh. 5 - Prob. 5.88EPCh. 5 - Prob. 5.89EPCh. 5 - Prob. 5.90EPCh. 5 - Assign common names to each of the esters in...Ch. 5 - Prob. 5.92EPCh. 5 - Prob. 5.93EPCh. 5 - Assign an IUPAC name to each of the following...Ch. 5 - Draw a structural formula for each of the...Ch. 5 - Prob. 5.96EPCh. 5 - Prob. 5.97EPCh. 5 - Prob. 5.98EPCh. 5 - Prob. 5.99EPCh. 5 - Prob. 5.100EPCh. 5 - Prob. 5.101EPCh. 5 - How many carbon atoms are present in a molecule of...Ch. 5 - Prob. 5.103EPCh. 5 - Prob. 5.104EPCh. 5 - Prob. 5.105EPCh. 5 - Prob. 5.106EPCh. 5 - Prob. 5.107EPCh. 5 - Prob. 5.108EPCh. 5 - Prob. 5.109EPCh. 5 - Prob. 5.110EPCh. 5 - Prob. 5.111EPCh. 5 - Prob. 5.112EPCh. 5 - Prob. 5.113EPCh. 5 - Prob. 5.114EPCh. 5 - Prob. 5.115EPCh. 5 - Prob. 5.116EPCh. 5 - Prob. 5.117EPCh. 5 - Prob. 5.118EPCh. 5 - Prob. 5.119EPCh. 5 - Prob. 5.120EPCh. 5 - Prob. 5.121EPCh. 5 - Prob. 5.122EPCh. 5 - Prob. 5.123EPCh. 5 - Prob. 5.124EPCh. 5 - Write the structural formulas of the reaction...Ch. 5 - Prob. 5.126EPCh. 5 - Prob. 5.127EPCh. 5 - Prob. 5.128EPCh. 5 - Prob. 5.129EPCh. 5 - Prob. 5.130EPCh. 5 - Prob. 5.131EPCh. 5 - Prob. 5.132EPCh. 5 - Prob. 5.133EPCh. 5 - Prob. 5.134EPCh. 5 - Prob. 5.135EPCh. 5 - Prob. 5.136EPCh. 5 - Prob. 5.137EPCh. 5 - Prob. 5.138EPCh. 5 - Prob. 5.139EPCh. 5 - Prob. 5.140EPCh. 5 - Prob. 5.141EPCh. 5 - Prob. 5.142EPCh. 5 - Prob. 5.143EPCh. 5 - Prob. 5.144EPCh. 5 - Prob. 5.145EPCh. 5 - Prob. 5.146EPCh. 5 - Prob. 5.147EPCh. 5 - Prob. 5.148EPCh. 5 - Draw a condensed structural formula for the...Ch. 5 - Draw a condensed structural formula for the...Ch. 5 - Prob. 5.151EPCh. 5 - Prob. 5.152EPCh. 5 - Prob. 5.153EPCh. 5 - Prob. 5.154EPCh. 5 - Prob. 5.155EPCh. 5 - Prob. 5.156EPCh. 5 - Prob. 5.157EPCh. 5 - Prob. 5.158EPCh. 5 - Prob. 5.159EPCh. 5 - Prob. 5.160EPCh. 5 - Prob. 5.161EPCh. 5 - Prob. 5.162EPCh. 5 - Prob. 5.163EPCh. 5 - Prob. 5.164EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,