
(a)
Interpretation:
The IUPAC name of reaction products when ethyl pentanoate undergoes ester hydrolysis under acidic conditions has to be written.
Concept Introduction:
Breaking of the carbon‑oxygen single bond present between the “acid part” and “alcohol part” is one of the important reactions of ester. This process of breaking the bond between the carbon‑oxygen is known as ester hydrolysis or saponification. The condition prevails in the reaction determines it as ester hydrolysis of saponification.
Ester hydrolysis takes place in ester when it is treated with strong acid or enzymes as catalyst. Reverse of esterification reaction is the ester hydrolysis.
Saponification is the reaction that ester undergoes when a strong base is used to give the product as
(a)
Answer to Problem 5.130EP
The IUPAC names of the products obtained are,
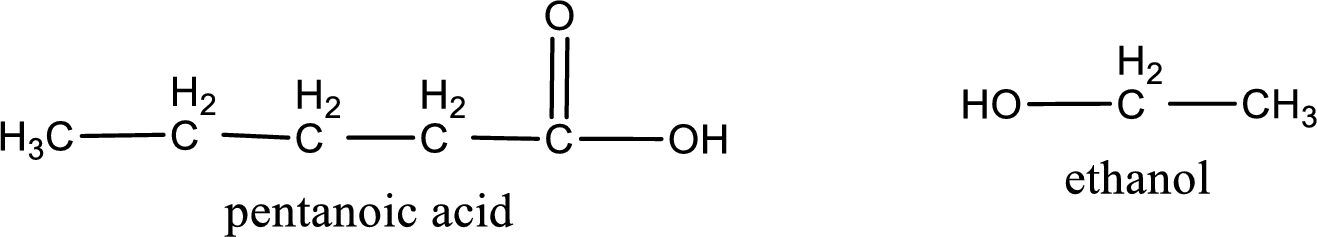
Explanation of Solution
Given name of ester is ethyl pentanoate. The structure of ethyl pentanoate can be given as,
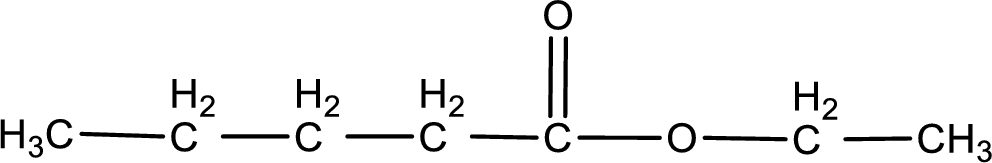
Under acidic conditions, esters undergo hydrolysis resulting in breakage of the carbon‑oxygen single bond that is present between the “acid part” and “alcohol part”. The product that is obtained on ester hydrolysis in acidic conditions is carboxylic acid and an alcohol. The complete reaction and the structure of the product obtained can be written as shown below,
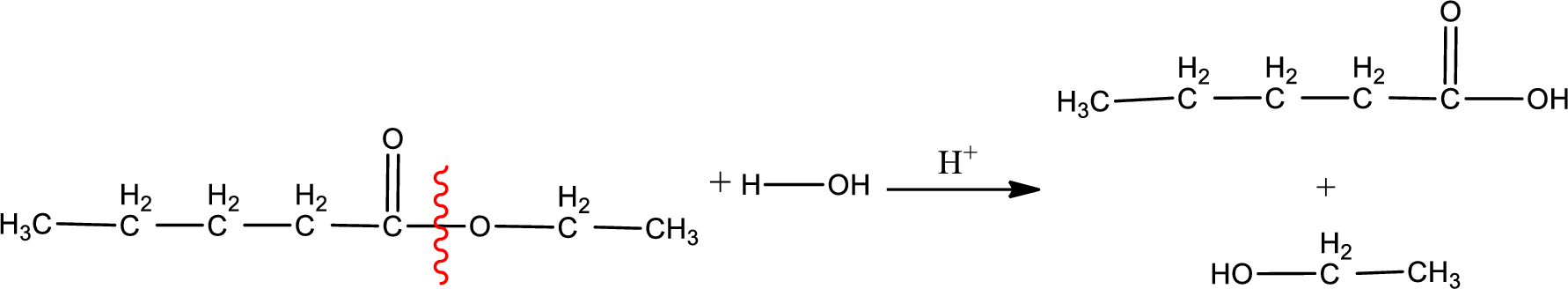
The IUPAC names of the product obtained can be given using
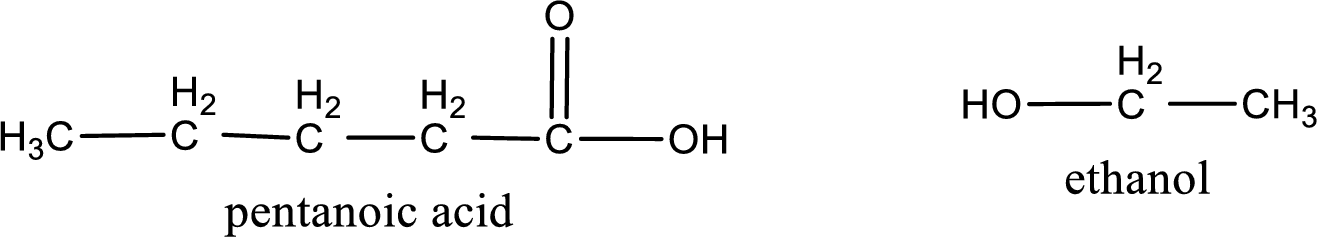
IUPAC names of the products obtained when ethyl pentanoate undergoes hydrolysis under acidic condition are written.
(b)
Interpretation:
The IUPAC name of reaction products when ethyl methanoate undergoes ester hydrolysis under acidic conditions has to be written.
Concept Introduction:
Breaking of the carbon‑oxygen single bond present between the “acid part” and “alcohol part” is one of the important reactions of ester. This process of breaking the bond between the carbon‑oxygen is known as ester hydrolysis or saponification. The condition prevails in the reaction determines it as ester hydrolysis of saponification.
Ester hydrolysis takes place in ester when it is treated with strong acid or enzymes as catalyst. Reverse of esterification reaction is the ester hydrolysis.
Saponification is the reaction that ester undergoes when a strong base is used to give the product as carboxylic acid salt and alcohol.
(b)
Answer to Problem 5.130EP
The structural formula and IUPAC names of the products obtained are,
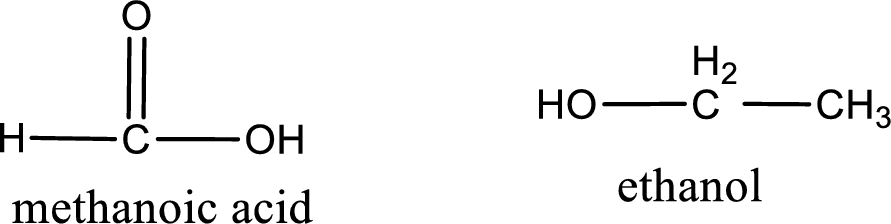
Explanation of Solution
Given name of ester is ethyl methanoate. The structure of ethyl methanoate can be given as,
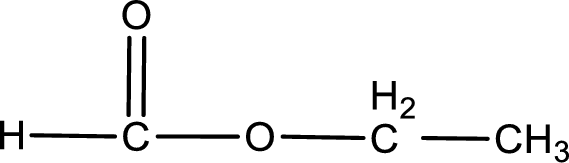
Under acidic conditions, esters undergo hydrolysis resulting in breakage of the carbon‑oxygen single bond that is present between the “acid part” and “alcohol part”. The product that is obtained on ester hydrolysis in acidic conditions is carboxylic acid and an alcohol. The complete reaction and the structure of the product obtained can be written as shown below,
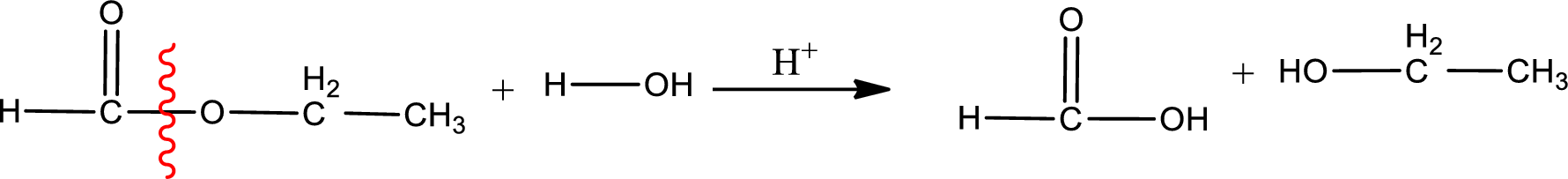
The IUPAC names of the product obtained can be given using IUPAC nomenclature of naming the compounds. The IUPAC names and the structure of the product obtained are,

IUPAC names of the products obtained when ethyl methanoate undergoes hydrolysis under acidic condition are written.
(c)
Interpretation:
The IUPAC name of reaction products when isopropyl pentanoate undergoes ester hydrolysis under acidic conditions has to be written.
Concept Introduction:
Breaking of the carbon‑oxygen single bond present between the “acid part” and “alcohol part” is one of the important reactions of ester. This process of breaking the bond between the carbon‑oxygen is known as ester hydrolysis or saponification. The condition prevails in the reaction determines it as ester hydrolysis of saponification.
Ester hydrolysis takes place in ester when it is treated with strong acid or enzymes as catalyst. Reverse of esterification reaction is the ester hydrolysis.
Saponification is the reaction that ester undergoes when a strong base is used to give the product as carboxylic acid salt and alcohol.
(c)
Answer to Problem 5.130EP
The structural formula and IUPAC names of the products obtained are,
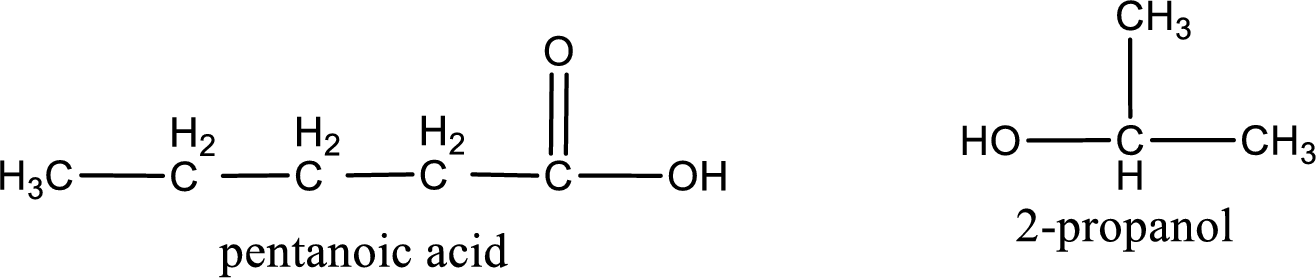
Explanation of Solution
Given name of ester is isopropyl pentanoate. The structure of isopropyl pentanoate can be given as,
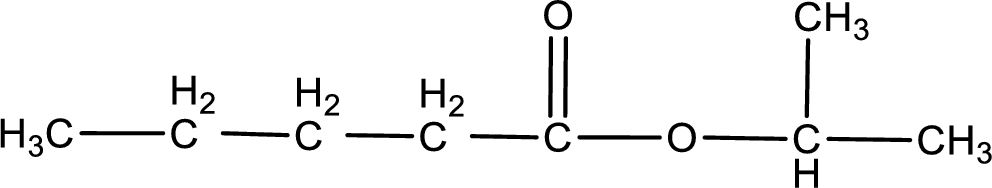
Under acidic conditions, esters undergo hydrolysis resulting in breakage of the carbon‑oxygen single bond that is present between the “acid part” and “alcohol part”. The product that is obtained on ester hydrolysis in acidic conditions is carboxylic acid and an alcohol. The complete reaction and the structure of the product obtained can be written as shown below,
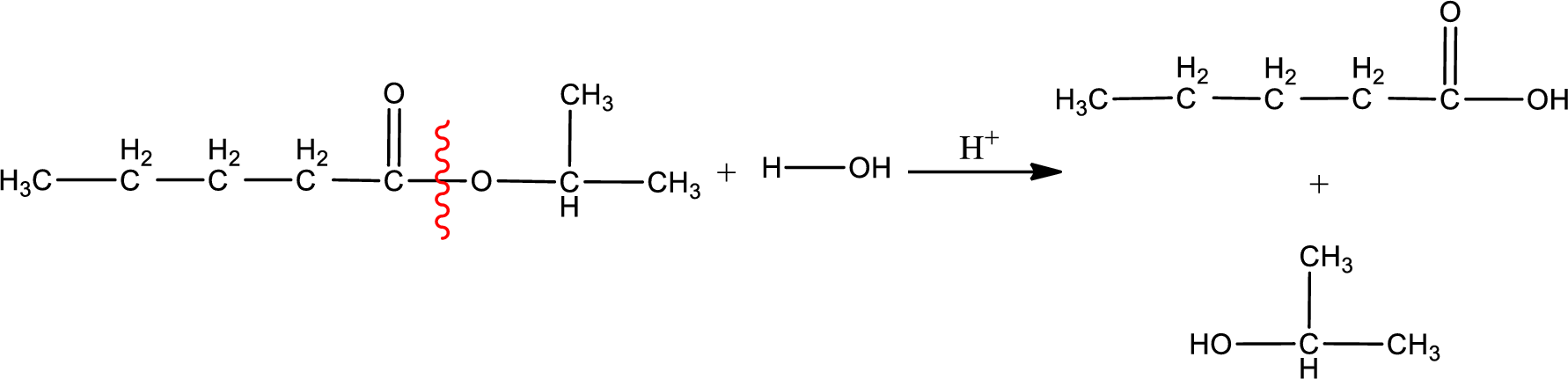
The IUPAC names of the product obtained can be given using IUPAC nomenclature of naming the compounds. The IUPAC names and the structure of the product obtained are,
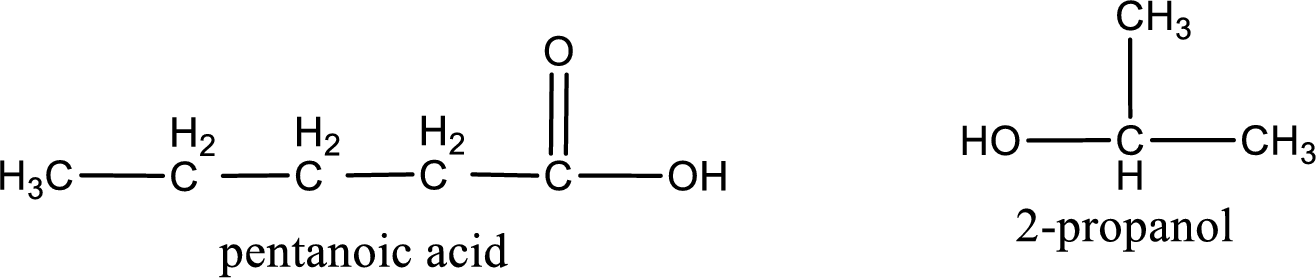
IUPAC names of the products obtained when isopropyl pentanoate undergoes hydrolysis under acidic condition are written.
(d)
Interpretation:
The IUPAC name of reaction products when isopropyl methanoate undergoes ester hydrolysis under acidic conditions has to be written.
Concept Introduction:
Breaking of the carbon‑oxygen single bond present between the “acid part” and “alcohol part” is one of the important reactions of ester. This process of breaking the bond between the carbon‑oxygen is known as ester hydrolysis or saponification. The condition prevails in the reaction determines it as ester hydrolysis of saponification.
Ester hydrolysis takes place in ester when it is treated with strong acid or enzymes as catalyst. Reverse of esterification reaction is the ester hydrolysis.
Saponification is the reaction that ester undergoes when a strong base is used to give the product as carboxylic acid salt and alcohol.
(d)
Answer to Problem 5.130EP
The structural formula and IUPAC names of the products obtained are,
Explanation of Solution
Given name of ester is isopropyl methanoate. The structure of isopropyl methanoate can be given as,
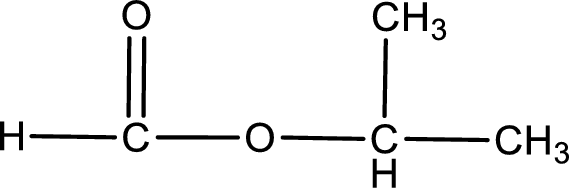
Under acidic conditions, esters undergo hydrolysis resulting in breakage of the carbon‑oxygen single bond that is present between the “acid part” and “alcohol part”. The product that is obtained on ester hydrolysis in acidic conditions is carboxylic acid and an alcohol. The complete reaction and the structure of the product obtained can be written as shown below,
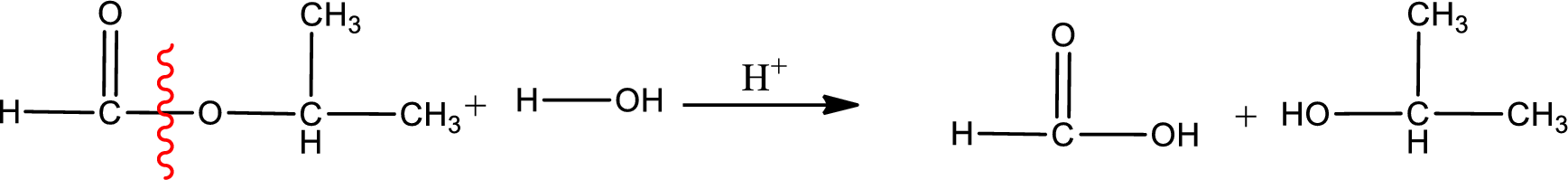
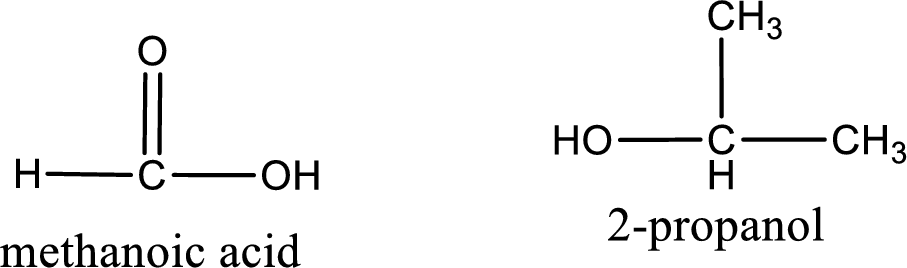
The IUPAC names of the product obtained can be given using IUPAC nomenclature of naming the compounds. The IUPAC names and the structure of the product obtained are,
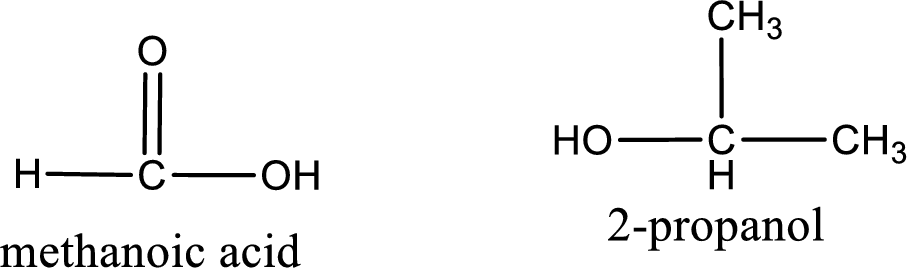
IUPAC names of the products obtained when isopropyl methanoate undergoes hydrolysis under acidic condition are written.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic And Biological Chemistry
- Relative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardPhenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward
- 12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward: Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forward
- Draw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forward
- Answer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





