
(a)
Interpretation:
The structure of 2-chlorobutane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
Answer to Problem 5SSC
Explanation of Solution
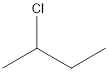
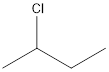
The given name of the compound is 2-chlorobutane.
The root name is “but” thus, there are 4 carbon atoms in the main chain. Prefix 2-chloro indicates the presence of chlorine atom on the 2nd position.
Thus, the structure of compound will be:
(b)
Interpretation:
The structure of 1, 3-difluorohexane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. For example, alcohol group will have suffix −ol, aldehyde will have suffix −al. If there is no functional group the suffix will be −ane. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
Answer to Problem 5SSC
Explanation of Solution
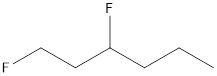
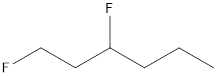
The given name of the compound is 1, 3-difluorohexane.
The root name is “hex” thus, there are 6 carbon atoms in the main chain. Prefix 3-difluoro indicates the presence of 2 fluorine atoms each on 1st and 3rd position.
Thus, the structure of compound will be:
(c)
Interpretation:
The structure of 1, 1, 1-trichloroethane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. For example, alcohol group will have suffix −ol, aldehyde will have suffix −al. If there is no functional group the suffix will be −ane. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
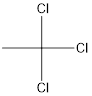
Answer to Problem 5SSC
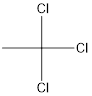
Explanation of Solution
The given name of the compound is 1, 1, 1-trichloroethane.
The root name is “eth” thus, there are 2 carbon atoms in the main chain. Prefix 1, 1, 1-trichloro indicates the presence of 3 chlorine atoms and all on the 1st position.
Thus, the structure of compound will be:
(d)
Interpretation:
The structure of 4-bromo-1-chlorobenzene needs to be drawn.
Concept introduction:
The aryl halides are named after determining the halide group present in it. Generally in organic compounds, on the benzene substitution of different groups result in the formation of different compounds. The carbon atoms in the ring are numbered first and then the sides groups are identified. The naming of the side groups is done by following the alphabetical order.
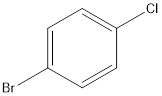
Answer to Problem 5SSC
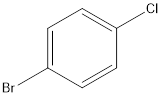
Explanation of Solution
The given name of the compound is 4-bromo-1-chlorobenzene.
According to the name, there is a benzene ring with one bromine and one chlorine atom at 4th and 1st position respectively.
Thus, the structure of compound will be:
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Campbell Biology (11th Edition)
Cosmic Perspective Fundamentals
Introductory Chemistry (6th Edition)
- At the end of the silica gel production process, color changes occur during drying. Explain these color changes.arrow_forwardIf CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.arrow_forwardWhen producing silica gel, color changes occur at the end of the drying process. Explain these color changes.arrow_forward
- Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forwardCorrect each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forward
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





