
a.
Interpretation:
The name of one alcohol,
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different
a.
Explanation of Solution
Generally, phenol is used as antiseptics. The structure of phenol is as follows:
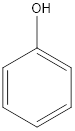
The functional group present in the compound is −OH group. Which is a hydroxyl group thus, the functional group is alcohol.
b.
Interpretation:
The name of one alcohol, amine or ether used in solvent in paint strippers needs to be determined.
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
b.
Explanation of Solution
The main ingredient used in the solvent in paint strippers is methylene chloride. Ethanol is also used which has −OH functional group thus, the name of one alcohol used in solvent in paint strippers is ethanol.
c.
Interpretation:
The name of one alcohol, amine or ether used in solvent in antifreeze needs to be determined.
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
c.
Explanation of Solution
Antifreeze is used to lower the freezing point of any substance. Generally, to reduce the freezing point of water, ethylene glycol is used.
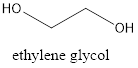
Thus, it contains two hydroxyl groups and it is an alcohol.
d.
Interpretation:
The name of one alcohol, amine or ether used in solvent in anesthetic needs to be determined.
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
d.
Explanation of Solution
A common anesthetic is diethyl ether. Its structure is represented as follows:
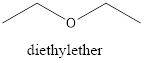
Thus, it is an example of ether.
e.
Interpretation:
The name of one alcohol, amine or ether used in dry production needs to be determined.
Concept introduction:
The functional group is the group present on the main carbon chain of an organic compound. It determines the chemical properties and the type of reactions an organic compound can show. The name of the group depends on the atoms present in the group for example an −OH group contains O and H atom thus, it is a hydroxyl group. The different functional groups are named according to IUPAC rule.
e.
Explanation of Solution
Ethanol can be used in the method of dry production. It is done by removing the water and other solvent from the given sample.
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
College Physics: A Strategic Approach (3rd Edition)
Applications and Investigations in Earth Science (9th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology in Focus (2nd Edition)
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
- Can I please get help with this?arrow_forward1. Draw structures corresponding to each of the following names [3 ONLY]: A. 2,2,2-trichloroethanal (chloral). B. trans-3-isopropylcyclohexanecarbaldehyde C. What is the correct structure for 2-hydroxyacetophenone? Circle the letter of your response. a C 0 OH OH OH HO b. H3C CH 0 H d OH D. Provide IUPAC names for each structure below. 0 H C-H 0 0 CH3 H NO₂ E. The substance formed on addition of water to an aldehyde or ketone is called a hydrate or a/an: a. vicinal diol b. geminal diol C. acetal d. ketalarrow_forwardAssign this spectrumarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





