
Concept explainers
(a)
Interpretation:
The given substitution reaction needs to be shown using the structural formulas of given reactants and products.
Concept introduction:
In the substitution reaction, one
Explanation of Solution
In the given reaction, 2-chloropropane and water undergoes substitution reaction to form 2-propanol and hydrogen chloride.
The reaction can be represented as follows:

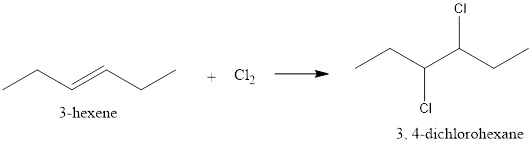
(b)
Interpretation:
The given addition reaction needs to be shown using the structural formulas of given reactants and products.
Concept introduction:
In an addition reaction, one molecule simply combines to other to form a larger molecule. There is no side product formed in the reaction.
Explanation of Solution
In the given reaction, 3-hexene and chlorine reacts to form 3, 4-dichlorohexane.
The reaction is represented as follows:
Chapter 22 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Cosmic Perspective Fundamentals
Campbell Biology: Concepts & Connections (9th Edition)
Human Anatomy & Physiology (2nd Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
- 3. Name this compound properly, including stereochemistry. H₂C H3C CH3 OH 4. Show the step(s) necessary to transform the compound on the left into the acid on the right. Bri CH2 5. Write in the product of this LiAlH4 Br H₂C OHarrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





