
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.SE, Problem 24VC
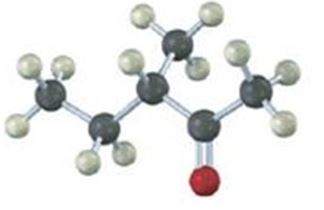
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethylmagnesium bromide. Is the product chiral? Is it optically active? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6) Fill in the missing Acid, pKa value, or conjugate base in the table below:
Acid
HCI
Approximate pK,
-7
Conjugate Base
H-C:
Hydronium (H₂O')
-1.75
H-O-H
Carboxylic Acids (RCOOH)
Ammonium (NH4)
9.24
Water (H₂O)
H-O-H
Alcohols (ROH)
RO-H
Alkynes
R--H
Amines
25
25
38
HO
5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least
acidic), and choose the justification(s) for each ranking.
(a)
OH
V
SH
я вон
CH
most acidic
(lowst pKa)
least acidic
(highest pKa)
Effect(s)
Effect(s)
Effect(s)
inductive effect O inductive effect O inductive effect
electronegativity electronegativity O electronegativity
resonance
polarizability
resonance
polarizability
O resonance
O polarizability
hybridization
Ohybridization
O hybridization
о
How negatively charged organic bases are formed.
Chapter 17 Solutions
Organic Chemistry
Ch. 17.1 - Give IUPAC names for the following compounds:Ch. 17.1 - Prob. 2PCh. 17.2 - The following data for isomeric four-carbon...Ch. 17.2 - Rank the following substances in order of...Ch. 17.2 - Prob. 5PCh. 17.3 - Prob. 6PCh. 17.4 - What reagent would you use to accomplish each of...Ch. 17.4 - Prob. 8PCh. 17.5 - Prob. 9PCh. 17.5 - Prob. 10P
Ch. 17.5 - Use the reaction of a Grignard reagent with a...Ch. 17.6 - How would you carry out the following...Ch. 17.6 - What products(s) would you expect from dehydration...Ch. 17.7 - What alcohols would give the following products on...Ch. 17.7 - What products would you expect from oxidation of...Ch. 17.8 - TMS ethers can be removed by treatment with...Ch. 17.9 - Show the mechanism for the reaction of...Ch. 17.11 - Prob. 18PCh. 17.11 - When the 1HNMR spectrum of an alcohol is run in...Ch. 17.SE - Give IUPAC names for the following compounds:Ch. 17.SE - Draw the structure of the carbonyl compound(s)...Ch. 17.SE - Prob. 22VCCh. 17.SE - Prob. 23VCCh. 17.SE - Name and assign R or S stereochemistry to the...Ch. 17.SE - Evidence for the intermediate carbocations in the...Ch. 17.SE - Acid-catalyzed dehydration of 2,...Ch. 17.SE - Prob. 27MPCh. 17.SE - Treatment of the following epoxide with aqueous...Ch. 17.SE - Prob. 29MPCh. 17.SE - Prob. 30MPCh. 17.SE - Identify the type of substitution mechanism (SN1,...Ch. 17.SE - The conversion of 3 alcohols into alkenes under...Ch. 17.SE - Prob. 33MPCh. 17.SE - The trimethylsilyl (TMS) protecting group is one...Ch. 17.SE - When the alcohol below is treated with POCI3 and...Ch. 17.SE - Phenols generally have lower pKa’s than...Ch. 17.SE - Give IUPAC names for the following compounds:Ch. 17.SE - Draw and name the eight isomeric alcohols with...Ch. 17.SE - Prob. 39APCh. 17.SE - Named bombykol, the sex pheromone secreted by the...Ch. 17.SE - Carvacrol is a naturally occurring substance...Ch. 17.SE - What Grignard reagent and what carbonyl compound...Ch. 17.SE - What carbonyl compounds would you reduce to...Ch. 17.SE - What carbonyl compounds might you start with to...Ch. 17.SE - Prob. 45APCh. 17.SE - What products would you obtain from reaction of...Ch. 17.SE - Prob. 47APCh. 17.SE - How would you prepare the following compounds from...Ch. 17.SE - Prob. 49APCh. 17.SE - What products would you expect to obtain from...Ch. 17.SE - Prob. 51APCh. 17.SE - Propose structures for alcohols that have the...Ch. 17.SE - Propose a structure consistent with the following...Ch. 17.SE - The 1HNMR spectrum shown is that of...Ch. 17.SE - A compound of unknown structure gave the following...Ch. 17.SE - Propose a structure for a compound C15H24O that...Ch. 17.SE - Prob. 57APCh. 17.SE - Prob. 58APCh. 17.SE - Rank the following substituted phenols in order of...Ch. 17.SE - Benzvl chloride can be converted into benzaldehvde...Ch. 17.SE - Prob. 61APCh. 17.SE - Prob. 62APCh. 17.SE - Prob. 63APCh. 17.SE - Prob. 64APCh. 17.SE - Prob. 65APCh. 17.SE - Prob. 66APCh. 17.SE - Dehydration of trans-2-methylcyclopentanol with...Ch. 17.SE - 2, 3-Dimethyl-2, 3-butanediol has the common name...Ch. 17.SE - As a rule, axial alcohols oxidize somewhat faster...Ch. 17.SE - Prob. 70APCh. 17.SE - A problem often encountered in the oxidation of...Ch. 17.SE - Identify the reagents a-f in the Following scheme:Ch. 17.SE - Prob. 73APCh. 17.SE - Prob. 74APCh. 17.SE - Compound A, C8H10O, has the IR and 1H NMR spectra...Ch. 17.SE - Prob. 76APCh. 17.SE - Prob. 77AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forwardO 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward
- (i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forwardMacmillan Learning Draw the acyl chloride that would give the ketone shown using the Friedel-Crafts acylation reaction. Select Draw Templates More с H о Cl 2Q Erase AICI₂arrow_forward
- Draw the complete mechanism for this reaction: .OH مدید OH H2SO4 + H₂O To save you some time, the starting material has been copied into the first drawing area. However, you will still need to add any other reactants or catalysts that take part in the reaction. ན ི.. OH Add/Remove step Х ด ك Click and drag to start drawing a structure.arrow_forward9:27 AM Tue Mar 4 ← Problem 64 of 15 #63% Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 0:0 :0: N. :0: :O :0: H H. :0: Select to Add Arrows O :0: H O :0: 0:0. S. H Select to Add Arrows S :0: :0: H Harrow_forwardOrder the following organic reactions by relative rate. That is, select '1' next to the reaction that will have the fastest initial rate, select '2' next to the reaction that will have the next fastest initial rate, and so on. If two reactions will have very similar initial rates, you can select the same number next to both. If a reaction will have zero or nearly zero initial rate, don't select a number and check the box in the table instead. Note: the "Nu" in these reactions means "a generic nucleophile." ملی CI :Nu 2 он 3 H Reaction Relative Rate (Choose one) ▼ Nu :CI: zero or nearly zero Nu :Nu bi (Choose one) zero or nearly zero : Nu لی Nu :H (Choose one) zero or nearly zeroarrow_forward
- 9:12 AM Tue Mar 4 66% Problem 38 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the product formed in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Br2 FeBrз H (+) Br: H : Br----FeBr3 く a SU 00 nd earrow_forwardUnder aqueous acidic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: ☐ : P Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. CN H₂O H₂O H+ H+ Click and drag to start drawing a structure. Хarrow_forwardOrganic bases have lone pairs of electrons that are capable of accepting protons. Lone pair electrons in a neutral or negatively charged species, or pi electron pairs. Explain the latter case (pi electron pairs).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY