
Concept explainers
What carbonyl compounds might you start with to prepare the Following compounds by Grignard reaction? List all possibilities.
(a) 2-Methyl-2-propanol
(b) 1-Ethylcyclohexanol
(c) 3-Phenyl-3-pentanol
(d) 2-Phenyl-2-pentanol
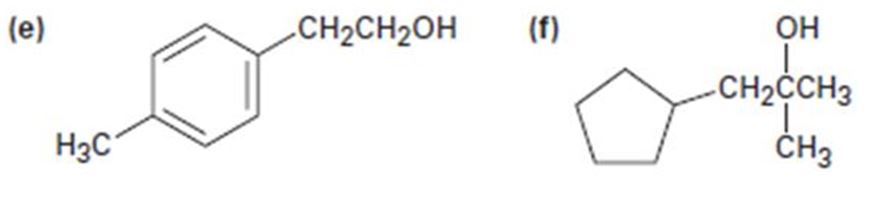
a) 2-Methyl-2-propanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol.
Answer to Problem 44AP
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
Explanation of Solution
A four carbon 30 alcohol is required. Hence a three carbon ketone (acetone) is treated with methylmagnesium bromide. In the case of esters two carbons will be provided by methylmagnesium bromide since esters require two molar equivalents of the reagent. Hence an ester of the two carbon acid (acetic acid) is chosen.
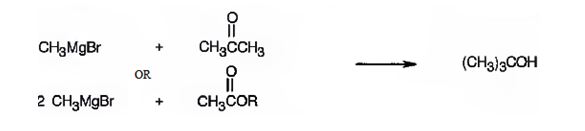
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
b) 1-Ethylcyclohexanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol.
Answer to Problem 44AP
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
Explanation of Solution
A six-membered cyclic 30 alcohol with ethyl group on C1 is required. Hence a six membered cyclic ketone (cyclohexanone) is treated with a two carbon Grignard reagent (ethylmagnesium bromide).
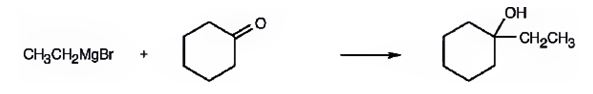
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
c) 3-Phenyl-3-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol.
Answer to Problem 44AP
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
Explanation of Solution
3-Phenyl-3-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C3. Hence an aromatic ketone (ethylphenyl ketone) is treated with ethylmagnesium bromide or the ester of benzoic acid is treated with two equivalents of ethylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with diethyl ketone.
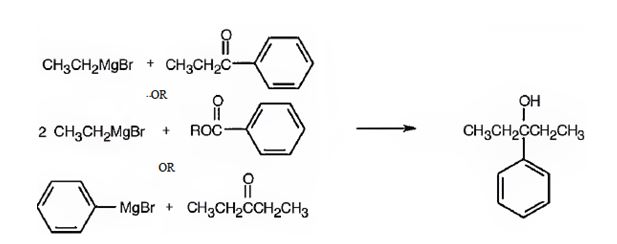
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
d) 2-Phenyl-2-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol.
Answer to Problem 44AP
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
Explanation of Solution
2-Phenyl-2-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C2. Hence an aromatic ketone like methylphenyl ketone is treated with propylmagnesium bromide or phenylpropyl ketone is treated methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with methylpropyl ketone.
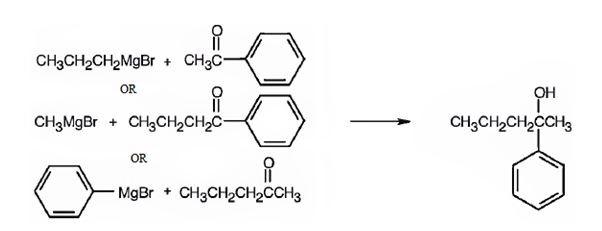
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
e)
Interpretation:
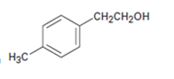
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-p-tolylethanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-(p-tolyl) ethanol.
Answer to Problem 44AP
2-(p-tolyl) ethanol can be prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
Explanation of Solution
2-(p-tolyl) ethanol is a 10 alcohol having a p-tolyl group attached to C2 of ethanol. Hence formaldehyde is required. The remaining part should come from the Grignard reagent. Hence formaldehyde is treated with p-tolylmethylmagnesium bromide.
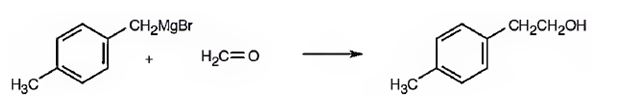
2-(p-tolyl) ethanol can prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
f)
Interpretation:
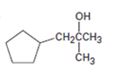
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Answer to Problem 44AP
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Explanation of Solution
1-Cyclopentyl-2-methyl-2-propanol is a 30 alcohol with a three carbon straight chain with a cyclopentyl group on C1 and –OH on C2. Hence cyclopentylmethyl methyl ketone is treated with methylmagnesium bromide or an ester of cyclopentylacetic acid is treated with two molar equivalents of methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence cyclopentylmethylmagnesium bromide is treated with acetone.
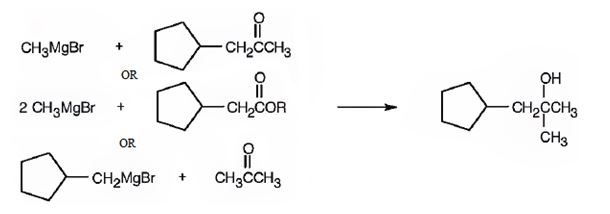
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forward
- Homework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forward
- Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

