
Concept explainers
A compound of unknown structure gave the following spectroscopic data:
Mass spectrum: M+=88.1
IR: 3600 cm-1
1ΗNMR: 1.4 δ (2 H, quartet, J=7 Hz); 1.2 δ (6H, singlet): 1.0 δ (1 H, singlet); 0.9 δ (3 H, triplet, J=7 Hz)
13CNMR: 74, 35, 27, 25 δ
(a) Assuming that the compound contains C and H but may or may not contain O, give three possible molecular formulas
(b) How many protons (H) does the compound contain?
(c) What
(d) How many carbons does the compound contain?
(e) What is the molecular formula of the compound?
(f) What is the structure of the compound?
(g) Assign peaks in the molecule’s 1HNMR spectrum corresponding to specific protons.
a) Three molecular formulas possible for the compound are to be given assuming that the compound contains C and H but may or may not contain oxygen.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600 cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
a) Three molecular formulas possible for the compound assuming that the compound contains C and H but may or may not contain oxygen.
Answer to Problem 55AP
a) Three molecular formulas possible for the compound assuming that the compound contains C and H and O are C5H12O, C4H8O2 and C3H4O3.
Explanation of Solution
a) The IR absorption at 3600cm-1 indicates the presence of an alcoholic group in the compound. Hence assuming the compound contains C, H and O, three possible formulas are possible for the compound with molecular mass 88. They are C5H12O, C4H8O2 and C3H4O3.
Three molecular formulas possible for the compound assuming that the compound contains C and H but may or may not contain oxygen are C5H12O, C4H8O2 and C3H4O3.
b) The number of protons in the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600 cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
b) The number of protons in the compound.
Answer to Problem 55AP
b) The compound has 12 protons.
Explanation of Solution
b) IN 1HNMR spectrum contain peaks accounting for the absorption for 12 protons in the molecule.
b) The compound has 12 protons.
c) The functional group(s) present in the compound is/are to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
c) The functional group(s) present in the compound.
Answer to Problem 55AP
c) The functional group in the compound is –OH (alcohol).
Explanation of Solution
c) In 13HNMR only four signals are observed. But 12 protons could not be accommodated on four carbons. At least five carbons are required. This justified by the 1HNMR spectrum which an absorption integrating to six protons present on two equivalent carbons. Hence five carbons are present in the molecule.
c) The functional group in the compound is –OH (alcohol).
d) The number of carbons in the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
d) The number of carbons in the compound.
Answer to Problem 55AP
d) The compound has 5 carbons.
Explanation of Solution
d) Its molecular formula has to be C5H12O as it is a five carbon alcohol with 12 hydrogen atoms.
d) The compound has 5 carbons.
e) Molecular formula of the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
e) Molecular formula of the compound.
Answer to Problem 55AP
e) Molecular formula of the compound is C5H12O.
Explanation of Solution
e) The six proton singlet at 1.2 δ can be attributed to two methyl groups attached to a carbon without any proton. The two proton triplet at 1.4 δ can be assigned to CH2 attached to a methyl which gives a triplet at 0.9 δ. The one proton singlet at 1.0 δ is due to the hydroxyl proton.
e) Molecular formula of the compound is C5H12O.
f) The structure of the compound is to be given.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To give:
f) The structure of the compound.
Answer to Problem 55AP
f) The structure of the compound is
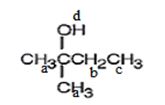
Explanation of Solution
f) Thus the structure of the alcohol is

The structure of the compound is

g) The peaks in the 1HNMR of the molecule are to be assigned to specific protons.
Interpretation:
A compound has the spectral data:
Mass spectrum: M+=88.1; IR=3600cm-1;
1HNMR: 1.4 δ (2H, quartet, J=7Hz); 1.2 δ (6H, singlet); 1.0 δ (1H, singlet); 0.9 δ (3H, triplet, J=7Hz)
13CNMR: 74, 35, 27, 25 δ
Concept introduction:
Molecular ion peak in the mass spectrum gives an idea about the relative molecular mass of the compound. From the molecular mass and knowing the atomic masses of C, H and O the possible molecular formulas for the compound can be arrived.
In IR the O-H stretching of alcohols and phenols are observed in the range 3200-3550 cm-1. In 1HNMR spectrum, the alcoholic proton absorption occurs in the range 3.4 δ - 4.5 δ while that of phenolic proton occur in the range 3.0 to 8.0. The absorption due to 10 alkyl group (CH3) is seen around 0.7 δ - 1.3 δ, that due to a 20 alkyl group (CH2) is seen around 1.2 δ - 1.6 δ while that due to 30 alkyl group (CH) is seen around 1.4 δ - 1.8 δ The multiplicity of a signal gives an idea about the protons present in the adjacent carbons.
In 13CNMR spectrum, the carbon bonded to –OH absorb in the range 50-80 δ. The primary alkyl carbon absorb in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To assign:
g) The peaks in the 1HNMR of the molecule to specific protons.
Answer to Problem 55AP
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
Explanation of Solution
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
g) 1.2 δ (6H, singlet) given by protons marked as ‘a’.
1.4 δ (2H, quartet, J=7Hz) given by protons marked as ‘b’.
0.9 δ (3H, triplet, J=7Hz) given by protons marked as ‘c’.
1.0 δ (1H, singlet) given by proton marked as‘d’.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

