
a)
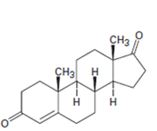
Interpretation:
Starting from testosterone how to prepare the compound shown is to be shown.
Concept introduction:
Pyridiniumchlorochromate in dichloromethane oxidizes 10 alcohols to
To show:
Starting from testosterone how to prepare the compound shown is to be stated.
b)
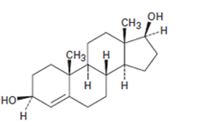
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
LiAlH4 in ether reduces unsaturated aldehydes, acids and esters to 10 alcohols. It reduces ketones to 20 alcohols. The double bond remains unaffected during the reduction.
To state:
Starting from testosterone how to prepare the compound shown.
c)
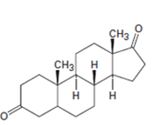
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
Pyridiniumchlorochromate in dichloromethane oxidizes 10 alcohols to aldehydes and 20 alcohols to ketones. H2, Pd/C can reduce the double bond in a compound without affecting aldehydic or keto group present in the compound.
To state:
Starting from testosterone how to prepare the compound shown.
d)
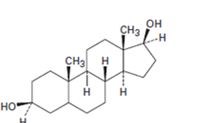
Interpretation:
Starting from testosterone how to prepare the compound shown is to be stated.
Concept introduction:
LiAlH4 in ether reduces unsaturated aldehydes, acids and esters to 10 alcohols. It reduces ketones to 20 alcohols. The double bond remains unaffected during the reduction. The double bond can be reduced using H2, Pd/C.
To state:
Starting from testosterone how to prepare the compound shown.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT





