
Concept explainers
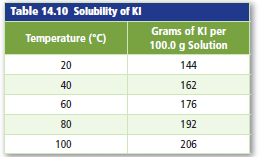
Graph Table 14.10 shows solubility data that was collectedin an experiment. Plot a graph of the molarity ofKI versus temperature. What is the solubility of KI at55°C?
Interpretation:
The solubility of potassium iodide (KI) at 55 °Chave to be calculated.
Concept introduction:
Solubility: Solubility can be expressed as maximum amount of solute that can dissolve in known amount of solvent at a given temperature and pressure. Solubility depends upon the temperature of the solvent. This is why many substances are more soluble at high temperatures.
Answer to Problem 116A
The solubility of potassium iodideat 55 °C is almost 10.5 M.
Explanation of Solution
Molarity of the KI solution at different temperatures have to calculate first.
- Molarity of the KI solution at20°C
- Molarity of the KI solution at 40°C
- Molarity of the KI solution at 60°C
- Molarity of the KI solution at 80°C
- Molarity of the KI solution at 100°C
Plotting the values of solubilities in molarity against temperature, we got
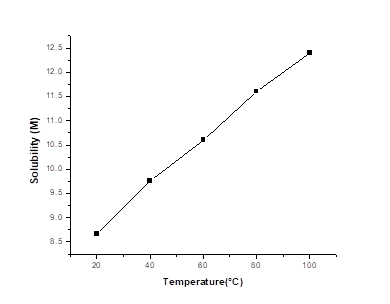
From the graph we can get the values of the solubility at different temperature. At 55°C, the solubility of KI is almost10.5 M.
Chapter 14 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Genetic Analysis: An Integrated Approach (3rd Edition)
Chemistry: The Central Science (14th Edition)
Chemistry: Structure and Properties (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





