
Interpretation:
Vapor pressure of solution is lower than pure solvent, needs to be described on particle basis.
Concept introduction:
Solution can be defined as a homogenous mixture that consist of two components solute and solvent.
Solvent: Solvent can be defined as a component that dissolves other component.
Solute: Solute can be defined as a component that is dissolved in the other component, solvent.
During solution formation, solute and solvent particles collide with each other, so that the solute gets dissolves in solvent.
Vapor pressure:
Vapor pressure is defined as the pressure exerted by a liquid particle that have entered the gaseous state in a closed container.
Answer to Problem 42SSC
Solution consists of two components- solute and solvent, so in a solution, solvent contains a solute, so lesser solventparticles occupy the surface and lesser particlesescape into the gaseous state that’s why vapor pressure of solution is lower than pure solvent
Vapor pressure is defined as the pressure exerted by a liquid particle that have entered the gaseous state in a closed container. When a non-volatile solute is added in solvent, it lowers the vapor pressure of the solvent. The particles escape the liquid phase at its surface that lowers the vapor pressure while in a pure solvent, the particles occupied the whole surface area, that is more particle will escape in gaseous state and vapor pressure increases.
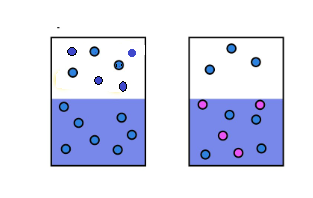
Pure solvent Solution(solute+solvent)
Explanation of Solution
Vapor pressure is defined as the pressure exerted by a liquid particle that have entered the gaseous state in a closed container. When a non-volatile solute is added in solvent, it lowers the vapor pressure of the solvent. The particles escape the liquid phase at its surface that lowers the vapor pressure while in a pure solvent, the particles occupied the whole surface area, that is more particle will escape in gaseous state and vapor pressure increases.

Pure solvent Solution(solute+solvent)
Chapter 14 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Campbell Essential Biology (7th Edition)
College Physics: A Strategic Approach (3rd Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- 3. Synthesize the following synthon from the indicated starting material. i HO.arrow_forwardIdentifying the stereochemistry of natural Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule H O-C-CH2 H3N. HN N H C=O common name (not the IUPAC name) NH3 ☐ H3N H ☐ CH2 Xarrow_forward> Draw the structure of alanine at pH 1.2. Click and drag to start drawing a structure.arrow_forward
- Understanding the general acid-base properties of amino acids O Proteins Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule The solution is... 010 H3N-CH-C-OH CH HO CH3 O acidic O basic neutral O (unknown) H3N HO 0 O acidic O basic neutral ○ (unknown) H3N-CH-C-O CH2 CH3-CH-CH3 O acidic O basic Oneutral ○ (unknown) O= X H2N-CH-C-O CH3 CH CH3 acidic O basic O neutral ○ (unknown) ? 000arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule 0=0 H3N-CH-C-o HO CH2 OH The solution is... O acidic O basic O neutral O (unknown) H₂N acidic O basic O neutral ○ (unknown) + H3N O OH O acidic O basic O neutral O (unknown) H2N-CH-C-O CH3 O acidic O basic neutral ○ (unknown) X ? olo HEarrow_forwardRecognizing ampli Draw an a amino acid with a methyl (-CH3) side chain. Explanation Check Click and drag to start drawing a structure. X Carrow_forward
- Write the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forwardく Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forward
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





