
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.SE, Problem 72AP
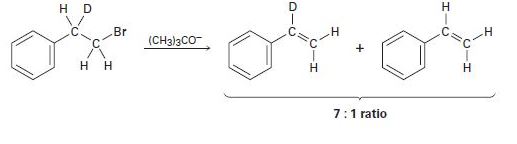
Treatment of 1-bromo-2-deuterio-2-phenylethane with strong base leads to a mixture of deuterated and nondeuterated phenylethylenes in an approximately 7:1 ratio. Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
help
helpME
Draw the major product of
this reaction. Ignore inorganic
byproducts.
Assume that the water side
product is continuously
removed to drive the reaction
toward products.
(CH3)2NH,
TSOH
Drawing
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Prob. 1PCh. 11.2 - Prob. 2PCh. 11.2 - Prob. 3PCh. 11.3 - Prob. 4PCh. 11.3 - Prob. 5PCh. 11.3 - Rank the following compounds in order of their...Ch. 11.3 - Organic solvents like benzene, ether, and...Ch. 11.4 - Prob. 8PCh. 11.4 - Prob. 9PCh. 11.4 - Prob. 10P
Ch. 11.5 - Rank the following substances in order of their...Ch. 11.5 - 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1...Ch. 11.5 - Prob. 13PCh. 11.6 - Review the mechanism of geraniol biosynthesis...Ch. 11.7 - Prob. 15PCh. 11.7 - What alkyl halides might the following alkenes...Ch. 11.8 - Prob. 17PCh. 11.8 - Prob. 18PCh. 11.9 - Prob. 19PCh. 11.12 - Prob. 20PCh. 11.SE - Prob. 21VCCh. 11.SE - From what alkyl bromide was the following alkyl...Ch. 11.SE - Prob. 23VCCh. 11.SE - Prob. 24VCCh. 11.SE - Prob. 25MPCh. 11.SE - Prob. 26MPCh. 11.SE - Prob. 27MPCh. 11.SE - Prob. 28MPCh. 11.SE - Prob. 29MPCh. 11.SE - Prob. 30MPCh. 11.SE - Prob. 31MPCh. 11.SE - Prob. 32MPCh. 11.SE - Metabolism of S-adenosylhomocysteine (Section...Ch. 11.SE - Reaction of iodoethane with CN- yields a small...Ch. 11.SE - One step in the urea cycle for ridding the body of...Ch. 11.SE - Prob. 36MPCh. 11.SE - Prob. 37MPCh. 11.SE - Propose a mechanism for the following reaction, an...Ch. 11.SE - Prob. 39APCh. 11.SE - The following Walden cycle has been carried out....Ch. 11.SE - Prob. 41APCh. 11.SE - Which reactant in each of the following pairs is...Ch. 11.SE - Prob. 43APCh. 11.SE - Prob. 44APCh. 11.SE - Prob. 45APCh. 11.SE - Prob. 46APCh. 11.SE - Prob. 47APCh. 11.SE - Prob. 48APCh. 11.SE - Propose structures for compounds that fit the...Ch. 11.SE - What products would you expect from the reaction...Ch. 11.SE - Prob. 51APCh. 11.SE - Prob. 52APCh. 11.SE - Prob. 53APCh. 11.SE - Prob. 54APCh. 11.SE - Prob. 55APCh. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Prob. 58APCh. 11.SE - Prob. 59APCh. 11.SE - Ethers can often be prepared by SN2 reaction of...Ch. 11.SE - Show the stereochemistry of the epoxide (see...Ch. 11.SE - Prob. 62APCh. 11.SE - In addition to not undergoing substitution...Ch. 11.SE - The tosylate of (2R, 3S)-3-phenyl-2-butanol...Ch. 11.SE - Prob. 65APCh. 11.SE - Prob. 66APCh. 11.SE - Prob. 67APCh. 11.SE - Prob. 68APCh. 11.SE - Prob. 69APCh. 11.SE - (S)-2-Butanol slowly racemizes on standing in...Ch. 11.SE - Reaction of HBr with (R)-3-methyl-3-hexanol leads...Ch. 11.SE - Treatment of 1-bromo-2-deuterio-2-phenylethane...Ch. 11.SE - Prob. 73APCh. 11.SE - Prob. 74APCh. 11.SE - In light of your answer to Problem 11-74, explain...Ch. 11.SE - Prob. 76APCh. 11.SE - Compound X is optically inactive and has the...Ch. 11.SE - When a primary alcohol is treated with...Ch. 11.SE - Prob. 79APCh. 11.SE - Amines are converted into alkenes by a two-step...Ch. 11.SE - The antipsychotic drug flupentixol is prepared by...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
- Which compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forwardHAND DRAWarrow_forward
- Predict the major products of the following organic reaction: Some important notes: Δ CN ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. ONO reaction. Click and drag to start drawing a structure.arrow_forwardThe following product was made from diethyl ketone and what other reagent(s)? £ HO 10 2-pentyne 1-butyne and NaNH2 ☐ 1-propanol ☐ pyridine butanal ☐ pentanoatearrow_forwardWhich pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY