
a)
Interpretation:
The actual product has to be identified.
Concept introduction:
Elimination reaction:An elimination reaction is removal of two substituents in a molecule and forms
E1 elimination:
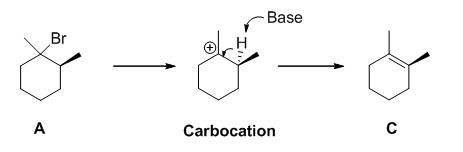
E2 elimination:Alkyl halide forms an alkene from the abstraction of the proton from the β-carbon atom followed by elimination of the bromine in a single step.
b)
Interpretation:
The actual product has to be identified.
Concept introduction:
Elimination reaction:An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E2 reaction. When two substituents are removed from the molecule in two steps is called E1 reaction.
E1 elimination: Alkyl halide forms carbocation by the removal of bromine followed by the abstraction of the proton from the β-carbon atom in two steps which leads to the product as an alkene

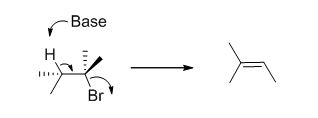
E2 elimination:Alkyl halide forms an alkene from the abstraction of the proton from the β-carbon atom followed by elimination of the bromine in a single step.

c)
Interpretation:
The actual product has to be identified.
Concept introduction:
Elimination reaction:An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E2 reaction. When two substituents are removed from the molecule in two steps is called E1 reaction.
E1 elimination: Alkyl halide forms carbocation by the removal of bromine followed by the abstraction of the proton from the β-carbon atom in two steps which leads to the product as an alkene

E2 elimination:Alkyl halide forms an alkene from the abstraction of the proton from the β-carbon atom followed by elimination of the bromine in a single step.

Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry
- What will the enolate for this be using LDA, THF, and cold temperatures? What will it be using NaOEt at rt?arrow_forwardHelp me solve this problem.arrow_forwardDraw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forward
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

