
Interpretation:
The Newman projection has to be drawn and the reason has to be explained for the formation of more trans product.
Concept introduction:
Conformations: Rotation about C-C single bonds allows a compound to adopt a variety of possible three-dimensional shapes.
Newman projections: The new conformations of compounds can be drawn and analyzed by Newman projections. A Newman projection visualizes different conformations of Carbon-carbon
The angle between two hydrogens of a Newman projection is called as dihedral angle or torsional angle. This dihedral angle changes as the C-C bond rotates. Two conformations with special attentions are staggered and eclipsed conformation. Staggered conformation is the lowest in energy and the eclipsed conformation is the highest in energy.
For example,

Anti-conformation: The conformation with a dihedral angle of is called anti-conformation.
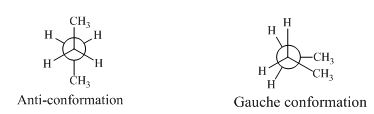
The two methyl groups achieve maximum separation from each other. In other, methyl groups are closer to each other; their electron clouds are repelling each other, causing an increase in energy. This unfavorable interaction is called gauche interaction.
E2 elimination:
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry
- What chemical has the common name "lye"? Pick one of the 3 esters and show the hydrolysis mechanism to make a carboxylic acid. The organic “R” should be used to limit the redrawing time of the entire molecule. * see imagearrow_forwardProvide the products for each reaction. There are two and they are not related. *see imagearrow_forwardd. a phenylal Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all levant stereochemistry. [three only] 0 A. B. CH3 Bra CH3COOH OH 1. Br₂, PBrz 2 H₂O 12arrow_forward
- 2arrow_forwardShow how the following conversions might be accomplished. Show all reagents and all intermediate ructures. More than one step may be required [2 ONLY]: A. B. ° C. OH 0 OH 0arrow_forwardA 20.3 mL sample of 0.263 M triethylamine, (C2H5)3N, is titrated with 0.252 M hydrochloric acid. (1) At the titration midpoint, the pH is . (2) At the equivalence point, the pH is .arrow_forward
- d. 3,4,5-trimethoxybenzoyl chloride . What is the order of decreasing reactivity towards nucleophilic acyl substitution for the arboxylic acid derivatives? (most reactive first) A. B. 0 0 O 0 0 H3C-C-O-C-CH3 H3C-C-N(CH3)2 H3C-C-OCH 3 (CH3)2CH-C-OCH3 I || ။ IV a. I, II, III, IV b. I, III, IV, II C. II, IV, III, I d. II, I, III, IV 0 0 0 0 0 R-C-O C-R R-C-NH2 R-C OR R-C-CI a. I, III, II, IV | 11 III IV b. II, III, I, IV c. III, II, I, IV d. IV, I, III, IIarrow_forwardB. d. a hydrate 4. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [4 ONLY]. A. CH₂OH PCC CH2Cl2 0 H KCN HCN 2arrow_forwardPropose a synthesis of the anti-inflammatory drug Ibuprofen from benzene. Show all reagents and all intermediate structures. Assume that ortho and para isomers can be separated. (CH3)2CHCH2 CH3 CHCOOH 1buprofen be requiredarrow_forward
- Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product: (a) KHC4H4O6arrow_forwardAnswer the following by equation 1. reactio of CH3MgBr with Acetone [CH3COCH3] 2. acetal formation reaction of acetaldehyde [CH3CHO] 3. preparation of ethylmethylether [C2H5OCH3] 4. the acidity of the carboxylic acid depends and affected by the substitutions on the rest of the acid molecule: draw 2 structures of acids to show the different effects on acidity by different subsarrow_forwardConsider the reaction sequence below to answer the following questions: 0 0 0 0 0 1. NaOEt, EtOH H3O* OEt OET 2 PhCH Br heat Ph + EtOH + CO₂ CHh B C A A. The starting material A in this reaction sequence is called a a. ẞ-keto ester b. a-carboethoxy ketone C. malonic ester d. acetoacetic ester B. Conversion of A into B is a type of reaction termed a. an acylation b. an enolation C. d. an alkylation a phenylation f reactionsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

