
a)
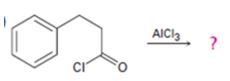
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The mechanism of the electrophilic substitution reaction is to be given. In the first step the electrophile is produced. In the second step the electrons of the
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
b)
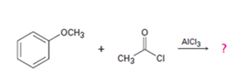
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The mechanism of the electrophilic substitution reaction is to be given. In the first step the electrophile is produced. In the second step the electrons of the aromatic ring attacks the elctrophile to give a resonance stabilized intermediate. In the last step the intermediate deprotonates to yield the product. The methoxyl group is an ortho and para orienting group.
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
c)
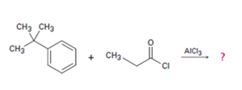
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to berovided.
Concept introduction:
The mechanism of the electrophilic substitution reaction is to be given. In the first step the electrophile is produced. In the second step the electrons of the aromatic ring attacks the elctrophile to give a resonance stabilized intermediate. In the last step the intermediate deprotonates to yield the product.
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

