
Interpretation:
The formation of
Concept introduction:
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E2 reaction. When two substituents are removed from the molecule in two steps is called E1 reaction.
E2 elimination:Alkyl halide forms an alkene from the abstraction of the proton from the β-carbon atom followed by elimination of the bromine in a single step.
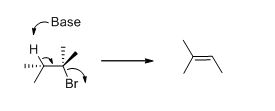
Given information:
The given compound is shown below,
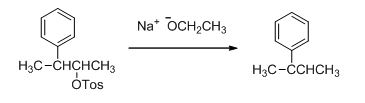
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry
- Determine the structures of the missing organic molecules in the following reaction: H+ O OH H+ + H₂O ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. X § ©arrow_forwardTable 1.1 Stock Standard Solutions Preparation. The amounts shown should be dissolved in 100 mL. Millipore water. Calculate the corresponding anion concentrations based on the actual weights of the reagents. Anion Amount of reagent (g) Anion Concentration (mg/L) 0.1649 Reagent Chloride NaCl Fluoride NaF 0.2210 Bromide NaBr 0.1288 Nitrate NaNO3 0.1371 Nitrite NaNO2 0.1500 Phosphate KH2PO4 0.1433 Sulfate K2SO4 0.1814arrow_forwardDraw the structure of the pound in the provided CO as a 300-1200 37(2), 11 ( 110, and 2.5 (20arrow_forward
- Please help me with # 4 and 5. Thanks in advance!arrow_forwardA small artisanal cheesemaker is testing the acidity of their milk before it coagulates. During fermentation, bacteria produce lactic acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and develop flavor. The cheesemaker has measured that the developing mixture contains lactic acid at an initial concentration of 0.025 M. Your task is to calculate the pH of this mixture and determine whether it meets the required acidity for proper cheese development. To achieve the best flavor, texture and reduce/control microbial growth, the pH range needs to be between pH 4.6 and 5.0. Assumptions: Lactic acid is a monoprotic acid H H :0:0: H-C-C H :0: O-H Figure 1: Lewis Structure for Lactic Acid For simplicity, you can use the generic formula HA to represent the acid You can assume lactic acid dissociation is in water as milk is mostly water. Temperature is 25°C 1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to include state symbols.…arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: :0 H. 0:0 :0: :6: S: :0: Select to Edit Arrows ::0 Select to Edit Arrows H :0: H :CI: Rotation Select to Edit Arrows H. < :0: :0: :0: S:arrow_forward
- 3:48 PM Fri Apr 4 K Problem 4 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Mg. :0: Select to Add Arrows :0: :Br: Mg :0: :0: Select to Add Arrows Mg. Br: :0: 0:0- Br -190 H 0:0 Select to Add Arrows Select to Add Arrows neutralizing workup H CH3arrow_forwardIarrow_forwardDraw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forward
- Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. Predict the major products of the following reaction. Explanation Q F1 A Check F2 @ 2 # 3 + X 80 F3 W E S D $ 4 I O H. H₂ 2 R Pt % 05 LL ee F6 F5 T <6 G Click and drag to start drawing a structure. 27 & A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acce Y U H DII 8 9 F10 4 J K L Z X C V B N M T H option command F11 P H commandarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. H :0: CH3 O: OH Q CH3OH2+ Draw Intermediate protonation CH3OH CH3OH nucleophilic addition H Draw Intermediate deprotonation :0: H3C CH3OH2* protonation H 0: H CH3 H.arrow_forwardPredicting the reactants or products of hemiacetal and acetal formation uentify the missing organic reactants in the following reaction: H+ X+Y OH H+ за Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. ? olo 18 Ar © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

