
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.SE, Problem 74AP
Interpretation Introduction
Interpretation:
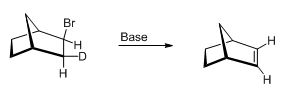
The molecular model has to be shown and explanation has to be given for the a syn elimination has occurred in the given reaction.
Concept introduction:
E2 elimination:
Given information:
The given compound is shown below,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a
4 shows scanning electron microscope (SEM) images of extruded
actions of packing bed for two capillary columns of different diameters,
al 750 (bottom image) and b) 30-μm-i.d. Both columns are packed with the
same stationary phase, spherical particles with 1-um diameter.
A) When the columns were prepared, the figure shows that the column with
the larger diameter has more packing irregularities. Explain this observation.
B) Predict what affect this should have on band broadening and discuss your
prediction using the van Deemter terms.
C) Does this figure support your explanations in application question 33?
Explain why or why not and make any changes in your answers in light of
this figure.
Figure 4 SEM images of
sections of packed columns
for a) 750 and b) 30-um-i.d.
capillary columns.³
fcrip
= ↓ bandwidth Il temp
32. What impact (increase, decrease, or no change) does each of the following conditions have on the individual
components of the van Deemter equation and consequently, band broadening?
Increase temperature
Longer column
Using a gas mobile phase
instead of liquid
Smaller particle stationary phase
Multiple Paths
Diffusion
Mass Transfer
34. Figure 3 shows Van Deemter plots for a solute molecule using different column inner diameters (i.d.).
A) Predict whether decreasing the column inner diameters increase or decrease bandwidth.
B) Predict which van Deemter equation coefficient (A, B, or C) has the greatest effect on increasing or
decreasing bandwidth as a function of i.d. and justify your answer.
Figure 3 Van Deemter plots for hydroquinone using different column inner diameters (i.d. in μm). The data was
obtained from liquid chromatography experiments using fused-silica capillary columns packed with 1.0-μm particles.
35
20
H(um)
큰 20
15
90
0+
1500
100
75
550
01
02
594
05
μ(cm/sec)
30
15
10
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Prob. 1PCh. 11.2 - Prob. 2PCh. 11.2 - Prob. 3PCh. 11.3 - Prob. 4PCh. 11.3 - Prob. 5PCh. 11.3 - Rank the following compounds in order of their...Ch. 11.3 - Organic solvents like benzene, ether, and...Ch. 11.4 - Prob. 8PCh. 11.4 - Prob. 9PCh. 11.4 - Prob. 10P
Ch. 11.5 - Rank the following substances in order of their...Ch. 11.5 - 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1...Ch. 11.5 - Prob. 13PCh. 11.6 - Review the mechanism of geraniol biosynthesis...Ch. 11.7 - Prob. 15PCh. 11.7 - What alkyl halides might the following alkenes...Ch. 11.8 - Prob. 17PCh. 11.8 - Prob. 18PCh. 11.9 - Prob. 19PCh. 11.12 - Prob. 20PCh. 11.SE - Prob. 21VCCh. 11.SE - From what alkyl bromide was the following alkyl...Ch. 11.SE - Prob. 23VCCh. 11.SE - Prob. 24VCCh. 11.SE - Prob. 25MPCh. 11.SE - Prob. 26MPCh. 11.SE - Prob. 27MPCh. 11.SE - Prob. 28MPCh. 11.SE - Prob. 29MPCh. 11.SE - Prob. 30MPCh. 11.SE - Prob. 31MPCh. 11.SE - Prob. 32MPCh. 11.SE - Metabolism of S-adenosylhomocysteine (Section...Ch. 11.SE - Reaction of iodoethane with CN- yields a small...Ch. 11.SE - One step in the urea cycle for ridding the body of...Ch. 11.SE - Prob. 36MPCh. 11.SE - Prob. 37MPCh. 11.SE - Propose a mechanism for the following reaction, an...Ch. 11.SE - Prob. 39APCh. 11.SE - The following Walden cycle has been carried out....Ch. 11.SE - Prob. 41APCh. 11.SE - Which reactant in each of the following pairs is...Ch. 11.SE - Prob. 43APCh. 11.SE - Prob. 44APCh. 11.SE - Prob. 45APCh. 11.SE - Prob. 46APCh. 11.SE - Prob. 47APCh. 11.SE - Prob. 48APCh. 11.SE - Propose structures for compounds that fit the...Ch. 11.SE - What products would you expect from the reaction...Ch. 11.SE - Prob. 51APCh. 11.SE - Prob. 52APCh. 11.SE - Prob. 53APCh. 11.SE - Prob. 54APCh. 11.SE - Prob. 55APCh. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Prob. 58APCh. 11.SE - Prob. 59APCh. 11.SE - Ethers can often be prepared by SN2 reaction of...Ch. 11.SE - Show the stereochemistry of the epoxide (see...Ch. 11.SE - Prob. 62APCh. 11.SE - In addition to not undergoing substitution...Ch. 11.SE - The tosylate of (2R, 3S)-3-phenyl-2-butanol...Ch. 11.SE - Prob. 65APCh. 11.SE - Prob. 66APCh. 11.SE - Prob. 67APCh. 11.SE - Prob. 68APCh. 11.SE - Prob. 69APCh. 11.SE - (S)-2-Butanol slowly racemizes on standing in...Ch. 11.SE - Reaction of HBr with (R)-3-methyl-3-hexanol leads...Ch. 11.SE - Treatment of 1-bromo-2-deuterio-2-phenylethane...Ch. 11.SE - Prob. 73APCh. 11.SE - Prob. 74APCh. 11.SE - In light of your answer to Problem 11-74, explain...Ch. 11.SE - Prob. 76APCh. 11.SE - Compound X is optically inactive and has the...Ch. 11.SE - When a primary alcohol is treated with...Ch. 11.SE - Prob. 79APCh. 11.SE - Amines are converted into alkenes by a two-step...Ch. 11.SE - The antipsychotic drug flupentixol is prepared by...
Knowledge Booster
Similar questions
- elow are experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.? 0.4 H (mm) 0.2 0.1- 0.3- 0 0.5 H (mm) 8.0 7.0 6.0 5.0 4.0- 3.0 T +++ 1.0 1.5 0 2.0 4.0 Flow Rate, u (cm/s) 6.0 8.0 Flow Rate, u (cm/s)arrow_forwardPredict the products of this organic reaction: + H ZH NaBH3CN H+ n. ? Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? + R H3O+ + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forwardExplain what is the maximum absorbance of in which caffeine absorbs?arrow_forward
- Explain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forwardProtecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forward
- Explanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning