
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.7, Problem 15P
Interpretation Introduction
Interpretation:
The elimination product of the following
a)
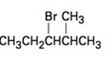
b)
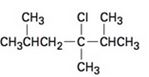
c)
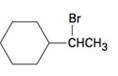
Concept introduction:
Elimination reactions usually results in the formation of mixture of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a
tricyclic product. Propose a structural formula for the product.
CN
heat
An intramolecular
Diels-Alder adduct
no Ai walkthroughs
What is the reaction mechanism for this? Can this even be done without a base?
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Prob. 1PCh. 11.2 - Prob. 2PCh. 11.2 - Prob. 3PCh. 11.3 - Prob. 4PCh. 11.3 - Prob. 5PCh. 11.3 - Rank the following compounds in order of their...Ch. 11.3 - Organic solvents like benzene, ether, and...Ch. 11.4 - Prob. 8PCh. 11.4 - Prob. 9PCh. 11.4 - Prob. 10P
Ch. 11.5 - Rank the following substances in order of their...Ch. 11.5 - 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1...Ch. 11.5 - Prob. 13PCh. 11.6 - Review the mechanism of geraniol biosynthesis...Ch. 11.7 - Prob. 15PCh. 11.7 - What alkyl halides might the following alkenes...Ch. 11.8 - Prob. 17PCh. 11.8 - Prob. 18PCh. 11.9 - Prob. 19PCh. 11.12 - Prob. 20PCh. 11.SE - Prob. 21VCCh. 11.SE - From what alkyl bromide was the following alkyl...Ch. 11.SE - Prob. 23VCCh. 11.SE - Prob. 24VCCh. 11.SE - Prob. 25MPCh. 11.SE - Prob. 26MPCh. 11.SE - Prob. 27MPCh. 11.SE - Prob. 28MPCh. 11.SE - Prob. 29MPCh. 11.SE - Prob. 30MPCh. 11.SE - Prob. 31MPCh. 11.SE - Prob. 32MPCh. 11.SE - Metabolism of S-adenosylhomocysteine (Section...Ch. 11.SE - Reaction of iodoethane with CN- yields a small...Ch. 11.SE - One step in the urea cycle for ridding the body of...Ch. 11.SE - Prob. 36MPCh. 11.SE - Prob. 37MPCh. 11.SE - Propose a mechanism for the following reaction, an...Ch. 11.SE - Prob. 39APCh. 11.SE - The following Walden cycle has been carried out....Ch. 11.SE - Prob. 41APCh. 11.SE - Which reactant in each of the following pairs is...Ch. 11.SE - Prob. 43APCh. 11.SE - Prob. 44APCh. 11.SE - Prob. 45APCh. 11.SE - Prob. 46APCh. 11.SE - Prob. 47APCh. 11.SE - Prob. 48APCh. 11.SE - Propose structures for compounds that fit the...Ch. 11.SE - What products would you expect from the reaction...Ch. 11.SE - Prob. 51APCh. 11.SE - Prob. 52APCh. 11.SE - Prob. 53APCh. 11.SE - Prob. 54APCh. 11.SE - Prob. 55APCh. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Order each of the following sets of compounds with...Ch. 11.SE - Prob. 58APCh. 11.SE - Prob. 59APCh. 11.SE - Ethers can often be prepared by SN2 reaction of...Ch. 11.SE - Show the stereochemistry of the epoxide (see...Ch. 11.SE - Prob. 62APCh. 11.SE - In addition to not undergoing substitution...Ch. 11.SE - The tosylate of (2R, 3S)-3-phenyl-2-butanol...Ch. 11.SE - Prob. 65APCh. 11.SE - Prob. 66APCh. 11.SE - Prob. 67APCh. 11.SE - Prob. 68APCh. 11.SE - Prob. 69APCh. 11.SE - (S)-2-Butanol slowly racemizes on standing in...Ch. 11.SE - Reaction of HBr with (R)-3-methyl-3-hexanol leads...Ch. 11.SE - Treatment of 1-bromo-2-deuterio-2-phenylethane...Ch. 11.SE - Prob. 73APCh. 11.SE - Prob. 74APCh. 11.SE - In light of your answer to Problem 11-74, explain...Ch. 11.SE - Prob. 76APCh. 11.SE - Compound X is optically inactive and has the...Ch. 11.SE - When a primary alcohol is treated with...Ch. 11.SE - Prob. 79APCh. 11.SE - Amines are converted into alkenes by a two-step...Ch. 11.SE - The antipsychotic drug flupentixol is prepared by...
Knowledge Booster
Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forwardWhat is the reaction mechanism for this?arrow_forward20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward
- 20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward20.21 Predict the major product formed by 1,4-addition of HCI to cyclopentadiene. 20.22 Draw structural formulas for the two constitutional isomers with the molecular for- mula C₂H,Br, formed by adding one mole of Br, to cyclopentadiene.arrow_forwardAdd substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward
- 20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward+ Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. Br Drawing Strong Base H Q Atoms, Bonds Charges and Rings Draw or tap a new bond to see suggestions. Remove Done 語 Reset Undo + Drag To Panarrow_forwardDraw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. + Drawing Į Strong Base H Br Q Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset 謂 Remove Done Drag To Pan +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
