
Concept explainers
(a)
Interpretation:
The possible resonance structures for the following skeleton structure have to be determined. Also, the resonance structure that is the most important has to be determined.
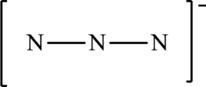
Concept Introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound that has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Estimate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
The formula to calculate formal charge of the atom is as follows:
Some molecules and ions do not have one unique Lewis structure. The Lewis structures that differ only in the placement of multiple bonds are called resonance structures.
Resonance structures are defined as a set of two or more Lewis structures that collectively describe the electronic bonding. The actual bonding is an average of the bonding in the resonance structures. Also, not all resonance structures contribute equally in every case. Resonance structures that have high formal charges or that place charges of the same sign on adjacent atoms do not contribute to the bonding.
(a)
Answer to Problem 9.69QE
The possible resonance structures are,
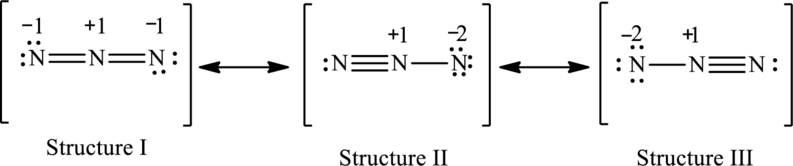
Structure I is more important.
Explanation of Solution
The given skeleton structure is,
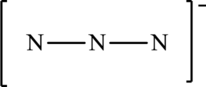
The resonance structures are as follows:
For structure I:
Substitute 5 for valence electrons, 4 for the number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
For structure II:
Substitute 5 for valence electrons, 2 for the number of lone pairs of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
For structure III:
Substitute 5 for valence electrons, 6 for the number of lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 2 for number of lone pairs of electrons and 6for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
The possible resonance structures with the formal charges are as follows:
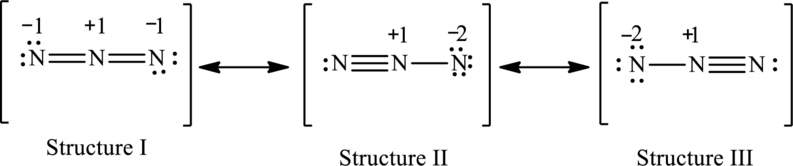
In these resonance structures, structure I have smallest formal charge because Lewis structures that have smallest formal charges are favored.
Hence, structure I is more important.
(b)
Interpretation:
The possible resonance structures for the following structure have to be determined. Also, the resonance structure that is the most important has to be determined.
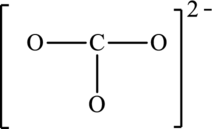
Concept introduction:
Refer to part (a)
(b)
Answer to Problem 9.69QE
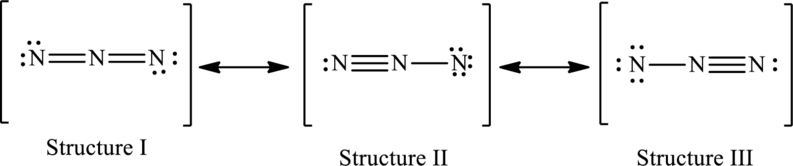
The possible resonance structures are as follows:
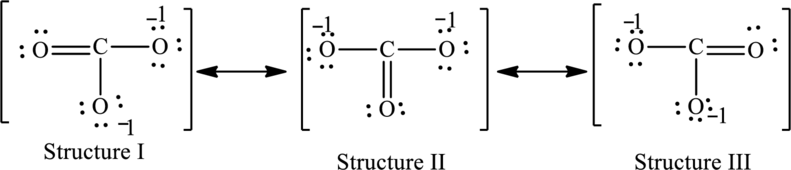
All structures are equally important.
Explanation of Solution
The given skeleton structure is,
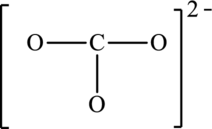
The resonance structures are as follows:
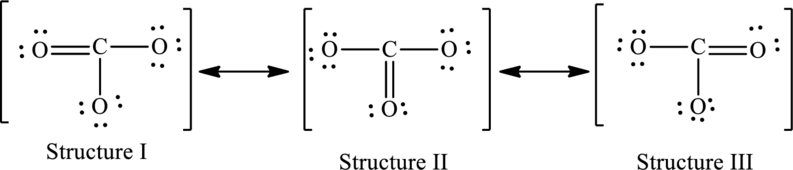
For structure I:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure II:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure III:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Possible resonance structures are as follows:
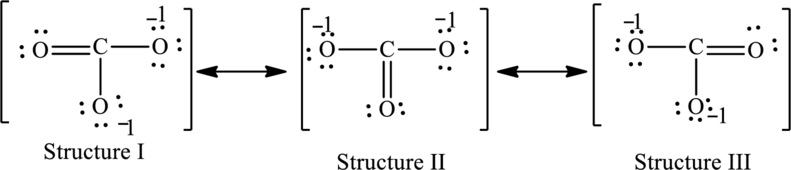
In these resonance structures, all the structures have the same formal charge. Hence, all the structures are equally important.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: Principles and Practice
- 13 Consider the "C NMR spectrum below. 140 120 100 80 60 40 20 20 PPM 0 The spectrum belongs to which one of the following constitutional isomers of the compound C,H12? Select the single best answer. Check ✓ G Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below. How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)? Check ×arrow_forward1 How many signals do you expect in the 'H NMR spectrum for this molecule? CI CI Cl Write the answer in the table below. Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum. 1 Number of signals in the 'H NMR spectrum. ☐ For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional H atoms to highlight in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at…arrow_forward
- wrtie the balanced equation and find the E° when the following half- reactions are combined Zn2+(aq) + 2e---> Zn(s) E°= -0.763V Ag+(aq) + e---> Ag (s) E°=+0.799Varrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forwardStudy this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forward
- how many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forwardan adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





