
Concept explainers
(a)
Interpretation:
The possible resonance structures for nitrate ion
Concept Introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound that has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Estimate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
The formula to calculate formal charge of the atom is as follows:
Some molecules and ions do not have one unique Lewis structure. The Lewis structures that differ only in the placement of multiple bonds are called resonance structures.
Resonance structures are defined as a set of two or more Lewis structures that collectively describe the electronic bonding. The actual bonding is an average of the bonding in the resonance structures. Also, not all resonance structures contribute equally in every case. Resonance structures that have high formal charges or that place charges of the same sign on adjacent atoms do not contribute to the bonding.
(a)
Answer to Problem 9.66QE
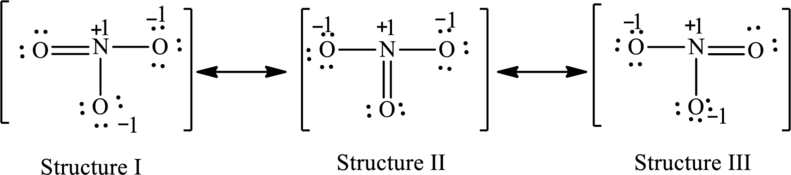
Possible resonance structures are as follows:
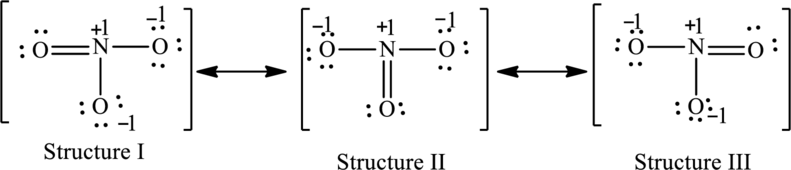
Explanation of Solution
The skeleton structure is,
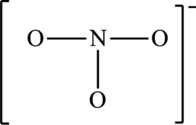
The resonance structures are as follows:
For structure I:
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure II:
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure III:
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
The possible resonance structures are as follows:
(b)
Interpretation:
The possible resonance structures for nitrous oxide
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 9.66QE
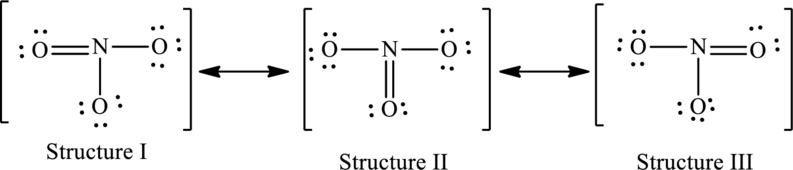
The possible resonance structures are,
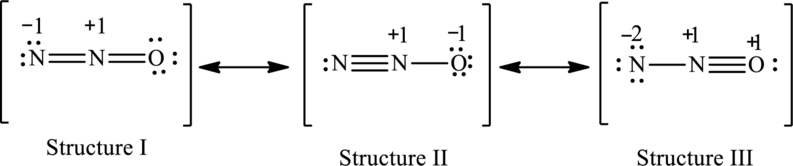
Explanation of Solution
The given skeleton structure is,
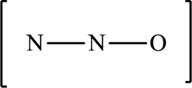
The resonance structures are as follows:
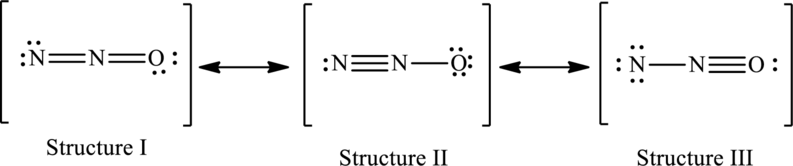
For structure I:
Substitute 5 for valence electrons, 4 for the number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on oxygen atom.
For structure II:
Substitute 5 for valence electrons, 2 for the number of lone pairs of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
For structure III:
Substitute 5 for valence electrons, 6 for the number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 6 for valence electrons, 2 for number of lone pairs of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on oxygen atom.
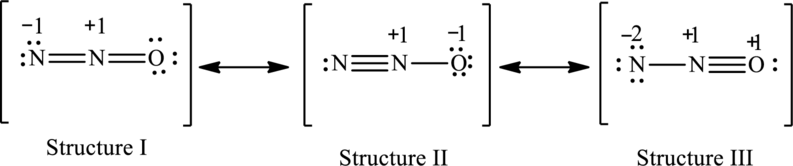
Possible resonance structures are as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: Principles and Practice
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



