
3-47 Answer true or false.
(a) The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion.
(b) In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound.
(c) The formula of aluminum oxide is AL2O3.
(d) Both copper(II) oxide and cupric oxide are acceptable names for CuO.
(e) The systematic name for Fe2O3 is iron(II) oxide.
(f) The systematic name for FeCO3 is ironcarbonate.
(g) The systematic name for NaH2PO4 is sodium dihydrogen phosphate.
(h) The systematic name for K,HPO4 is dipotassium hydrogen phosphate.
(i) The systematic name for Na20 is sodium oxide.
(j) The systematic name for PCL3 is potassium chloride.
(k) The formula of ammonium carbonate is NH4CO3.
(a)
Interpretation:
To analyse whether the given statement -The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion, is true or not.
Concept Introduction:
A covalent bond is formed by sharing of same number of electrons between two atoms to complete their octet. Atoms taking part in covalent bond formation may share one, two or three electron pairs thus forming single, double and triple bond respectively.
A binary covalent compound contains two kinds of atoms.
A covalent compound can be made of a atoms of the same element like Hydrogen, Oxygen etc, or it may be formed by atoms of different elements like
According to the rules for naming, binary covalent compound is named in this order-first the less electronegative element and then the more electronegative element.
Answer to Problem 3.47P
The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion. Thus, the statement is true.
Explanation of Solution
According to the rules for naming, binary covalent compound is named in this order-first the less electronegative element and then the more electronegative element.
For Example-
Electronic configuration of Beryllium, 2,2.
Valence shell has 2 electrons.
But to follow octet rule valance shall requires 8 electrons, for that it donates its 1 electron to two fluorine each.
On ejecting of 2 electrons now valence shell for Beryllium counts only 2 electrons, which completes its duplet. Hence it follows the octet rule.
Since, beryllium forms a cation it is written first, and Fluorine forming an anion is written later.
Therefore, the name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion.
(b)
Interpretation:
To analyse whether the given statement -In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound, is true or not.
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since, the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
Binary compounds may or may not have the same ratio in all the existing atoms. For example: carbon monoxide, CO contains 1 carbon and 1 oxygen atom but carbon dioxide
Answer to Problem 3.47P
In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound. Thus, the statement is true.
Explanation of Solution
Binary compounds may or may not have the same ratio in all the existing compound.
For example: carbon monoxide, CO contains 1 carbon and 1 oxygen atom but carbon dioxide
For example: In Iron (II) oxide, FeO, the ratio of Iron and Oxygen is 1:1 but in Iron (III) oxide,
Therefore. in naming binary ionic compounds, it is necessary to state the number of each ion present in the compound.
(c)
Interpretation:
To analyse whether the given statement -The formula of aluminium oxide is
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The formula of aluminium oxide is
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences. The naming of ionic compounds is done by crisscross method. Here, Al has +3 charge and oxygen has -2 charge thus, there will be 2 aluminum atoms and 3 oxygen atoms in the compound.
The formula of aluminium oxide is
(d)
Interpretation:
To analyse whether the given statement -Both copper (II) oxide and cupric oxide are acceptable names for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
Both copper (II) oxide and cupric oxide are acceptable names for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
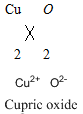
Therefore, both copper (II) oxide and cupric oxide are acceptable names for
(e)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Iron (II) oxide.
The systematic name for
The systematic name for
(f)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(g)
Interpretation:
To analyse whether the given statement -The systematic name for
dihydrogen phosphate is true or not.
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(h)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(i)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(j)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
A binary compound may or may not have same ratio of the atoms of the elements in the existing compounds.
The ratio of Phosphorous and chlorine in Phosphorous (III) Chloride is 1:3 Therefore, systematic name for
(k)
Interpretation:
To analyse whether the given statement -The formula of ammonium carbonate is
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The formula of ammonium carbonate is
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The formula of ammonium carbonate is
Want to see more full solutions like this?
Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
- ASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 IZ IN Molecule 4 Molecule 5 ZI none of the above ☐ Molecule 3 Х IN www Molecule 6 NH Garrow_forward
- Highlight each chiral center in the following molecule. If there are none, then check the box under the drawing area. There are no chiral centers. Cl Cl Highlightarrow_forwardA student proposes the following two-step synthesis of an ether from an alcohol A: 1. strong base A 2. R Is the student's proposed synthesis likely to work? If you said the proposed synthesis would work, enter the chemical formula or common abbreviation for an appropriate strong base to use in Step 1: If you said the synthesis would work, draw the structure of an alcohol A, and the structure of the additional reagent R needed in Step 2, in the drawing area below. If there's more than one reasonable choice for a good reaction yield, you can draw any of them. ☐ Click and drag to start drawing a structure. Yes No ロ→ロ 0|0 G Х D : ☐ பarrow_forwardटे Predict the major products of this organic reaction. Be sure to use wedge and dash bonds when necessary, for example to distinguish between different major products. ☐ ☐ : ☐ + NaOH HO 2 Click and drag to start drawing a structure.arrow_forward
- Shown below are five NMR spectra for five different C6H10O2 compounds. For each spectrum, draw the structure of the compound, and assign the spectrum by labeling H's in your structure (or in a second drawing of the structure) with the chemical shifts of the corresponding signals (which can be estimated to nearest 0.1 ppm). IR information is also provided. As a reminder, a peak near 1700 cm-1 is consistent with the presence of a carbonyl (C=O), and a peak near 3300 cm-1 is consistent with the presence of an O–H. Extra information: For C6H10O2 , there must be either 2 double bonds, or 1 triple bond, or two rings to account for the unsaturation. There is no two rings for this problem. A strong band was observed in the IR at 1717 cm-1arrow_forwardPredict the major products of the organic reaction below. : ☐ + Х ك OH 1. NaH 2. CH₂Br Click and drag to start drawing a structure.arrow_forwardNG NC 15Show all the steps you would use to synthesize the following products shown below using benzene and any organic reagent 4 carbons or less as your starting material in addition to any inorganic reagents that you have learned. NO 2 NC SO3H NO2 OHarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
