
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 5Q
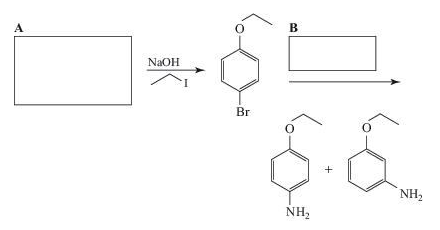
Complete the following synthesis:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
-AG|F=2E|V
3. Before proceeding with this problem you may want to glance at p. 466 of your textbook
where various oxo-phosphorus derivatives and their oxidation states are summarized.
Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14:
Acidic solution
-0.93
+0.38
-0.51 -0.06
H3PO4 →H4P206 H3PO3 H3PO2 → P→ PH3
-0.28
-0.50
→
-0.50
Basic solution
3-1.12
-1.57
-2.05 -0.89
PO HPO →→H2PO2 P PH3
-1.73
a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the
formation and reduction of H4P2O6 (-0.93/+0.38V). Calculate the values of AG's for both
processes; comment.
(3 points)
0.5 PH,
0.0
-0.5-
2 3 9 3
-1.5
-2.0
Pa
H,PO
H,PO
H,PO
-3
-1 0
2
4
Oxidation state, N
2
b) Frost diagram for phosphorus under acidic
conditions is shown. Identify possible
disproportionation and comproportionation processes;
write out chemical equations describing them. (2 points)
c) Elemental phosphorus tends to disproportionate under basic conditions. Use data in…
These two reactions appear to start with the same starting materials but result in different products. How do the chemicals know which product to form? Are both products formed, or is there some information missing that will direct them a particular way?
What would be the best choices for the missing reagents 1 and 3 in this synthesis?
1. PPh3
3
1
2
2. n-BuLi
• Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like.
• Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is.
• Note: if one of your reagents needs to contain a halogen, use bromine.
Explanation
Check
Click and drag to start drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priva
×
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - PRACTICE PROBLEM
21.1 If we examine Table 21.1, we...Ch. 21 - PRACTICE PROBLEM If we examine Table 21.1, we see...Ch. 21 - Prob. 3PPCh. 21 - PRACTICE PROBLEM
21.4 Predict the products of each...Ch. 21 - Prob. 5PPCh. 21 - PRACTICE PROBLEM
21.6 What are compounds A and B...Ch. 21 - Prob. 7PPCh. 21 - PRACTICE PROBLEM Outline a possible synthesis of...Ch. 21 - PRACTICE PROBLEM 1-Fluoro-2,4-dinitrobenzene is...Ch. 21 - PRACTICE PROBLEM
21.10 When o-chlorotoluene is...
Ch. 21 - PRACTICE PROBLEM When 2-bromo-1,3-dimethylbenzene...Ch. 21 - PRACTICE PROBLEM (a) Outline a step-by-step...Ch. 21 - Rank the following in order of increasing acidity.Ch. 21 - Prob. 14PCh. 21 - Prob. 15PCh. 21 - Describe a simple chemical test that could be used...Ch. 21 - Prob. 17PCh. 21 - Predict the product of the following reactions.Ch. 21 - 21.19 A synthesis of the β-receptor blocker called...Ch. 21 - Prob. 20PCh. 21 - When m-chlorotoluene is treated with sodium amide...Ch. 21 - Prob. 22PCh. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - Prob. 26PCh. 21 - Prob. 27PCh. 21 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Prob. 30PCh. 21 - Prob. 31PCh. 21 - 21.32 A compound X (C10H14O) dissolves in aqueous...Ch. 21 - 21.33 Compound Z (C5H10O) decolorizes bromine in...Ch. 21 - Explain why, in the case shown, the allyl group...Ch. 21 - In protic solvents the naphthoxide ion (I) is...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Prob. 40PCh. 21 - 21.41 Compound W was isolated from a marine...Ch. 21 - 21.42 Phenols generally are not changed on...Ch. 21 - 21.43 Open the molecular model file for benzyne...Ch. 21 - Which of the following would be the strongest...Ch. 21 - What products would you expect from the following...Ch. 21 - Prob. 3QCh. 21 - Prob. 4QCh. 21 - 21.5 Complete the following synthesis:
Ch. 21 - Prob. 6QCh. 21 - 21.7 Select the stronger acid.
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
Calculate the mass of NaCl in a 35-mL sample of a 1.3 M NaCl solution.
Introductory Chemistry (6th Edition)
Comprehend the molecular view of carbon dioxide gas. Concept Introduction: The chemical compounds can be classi...
Living By Chemistry: First Edition Textbook
Q1. Which wavelength of light has the highest frequency?
a) 10 nm
b) 10 mm
c) 1 nm
d) 1 mm
Chemistry: A Molecular Approach (4th Edition)
62. The sun is 30° above the horizon. It makes a 52-m-long shadow of a tall tree. How high is the tree?
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the products of this organic reaction: Explanation Check IN NaBH3CN H+ ? Click and drag to start drawing a structure. D 5 C +arrow_forwardPredict the products of this organic reaction: H3O+ + ? • Draw all the reasonable products in the drawing area below. If there are no products, because no reaction will occur, check the box under the drawing area. • Include both major and minor products, if some of the products will be more common than others. • Be sure to use wedge and dash bonds if you need to distinguish between enantiomers. No reaction. Click and drag to start drawing a structure. dmarrow_forwardIarrow_forward
- Draw the anti-Markovnikov product of the hydration of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for esc esc ☐ Explanation Check F1 1 2 F2 # 3 F3 + $ 14 × 1. BH THE BH3 2. H O NaOH '2 2' Click and drag to start drawing a structure. F4 Q W E R A S D % 905 LL F5 F6 F7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility < & 6 7 27 8 T Y U G H I F8 F9 F10 F11 F12 9 0 J K L P + // command option Z X C V B N M H H rol option commandarrow_forwardAG/F-2° V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: -0.93 +0.38 -0.50 -0.51 -0.06 H3PO4 →H4P206 →H3PO3 →→H3PO₂ → P → PH3 Acidic solution Basic solution -0.28 -0.50 3--1.12 -1.57 -2.05 -0.89 PO HPO H₂PO₂ →P → PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P206 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH P 0.0 -0.5 -1.0- -1.5- -2.0 H.PO, -2.3+ -3 -2 -1 1 2 3 2 H,PO, b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) H,PO 4 S Oxidation stale, Narrow_forward4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward
- 2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 3 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH3CN H+ N Click and drag to start drawing a structure. 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY