
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 18P
Predict the product of the following reactions.
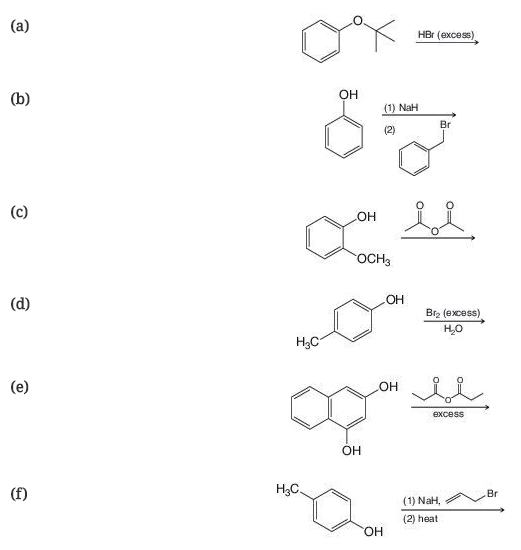
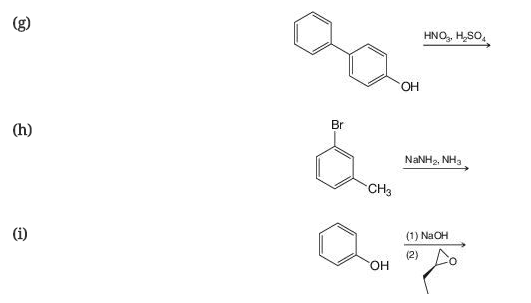
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
These are in the wrong boxes. Why does the one on the left have a lower molar mass than the one on the right?
SYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants.
Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the
mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for
that step's mechanism.
CI
b.
a.
Use acetylene (ethyne)
and any alkyl halide as
your starting materials
Br
C.
d.
"OH
OH
III.
OH
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - PRACTICE PROBLEM
21.1 If we examine Table 21.1, we...Ch. 21 - PRACTICE PROBLEM If we examine Table 21.1, we see...Ch. 21 - Prob. 3PPCh. 21 - PRACTICE PROBLEM
21.4 Predict the products of each...Ch. 21 - Prob. 5PPCh. 21 - PRACTICE PROBLEM
21.6 What are compounds A and B...Ch. 21 - Prob. 7PPCh. 21 - PRACTICE PROBLEM Outline a possible synthesis of...Ch. 21 - PRACTICE PROBLEM 1-Fluoro-2,4-dinitrobenzene is...Ch. 21 - PRACTICE PROBLEM
21.10 When o-chlorotoluene is...
Ch. 21 - PRACTICE PROBLEM When 2-bromo-1,3-dimethylbenzene...Ch. 21 - PRACTICE PROBLEM (a) Outline a step-by-step...Ch. 21 - Rank the following in order of increasing acidity.Ch. 21 - Prob. 14PCh. 21 - Prob. 15PCh. 21 - Describe a simple chemical test that could be used...Ch. 21 - Prob. 17PCh. 21 - Predict the product of the following reactions.Ch. 21 - 21.19 A synthesis of the β-receptor blocker called...Ch. 21 - Prob. 20PCh. 21 - When m-chlorotoluene is treated with sodium amide...Ch. 21 - Prob. 22PCh. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - Prob. 26PCh. 21 - Prob. 27PCh. 21 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Prob. 30PCh. 21 - Prob. 31PCh. 21 - 21.32 A compound X (C10H14O) dissolves in aqueous...Ch. 21 - 21.33 Compound Z (C5H10O) decolorizes bromine in...Ch. 21 - Explain why, in the case shown, the allyl group...Ch. 21 - In protic solvents the naphthoxide ion (I) is...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Prob. 40PCh. 21 - 21.41 Compound W was isolated from a marine...Ch. 21 - 21.42 Phenols generally are not changed on...Ch. 21 - 21.43 Open the molecular model file for benzyne...Ch. 21 - Which of the following would be the strongest...Ch. 21 - What products would you expect from the following...Ch. 21 - Prob. 3QCh. 21 - Prob. 4QCh. 21 - 21.5 Complete the following synthesis:
Ch. 21 - Prob. 6QCh. 21 - 21.7 Select the stronger acid.
Additional Science Textbook Solutions
Find more solutions based on key concepts
How many protons are in the nucleus of an atom of each element? a. Ti b. Li c. U d. Br e. F
Introductory Chemistry (6th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
1.4 Consider the two spheres shown here, one made of silver and the other of aluminum.
What is the mass of eac...
Chemistry: The Central Science (14th Edition)
A piston/cylinder arrangement has the piston loaded with outside atmospheric pressure and the piston mass to a ...
Fundamentals Of Thermodynamics
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
SSM An electric dipole consisting of charges of magnitude 1.50 nC separated by 6.20 m is in an electric field o...
Fundamentals of Physics Extended
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY