
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 35P
In protic solvents the naphthoxide ion (I) is alkylated primarily at position 1 (C-alkylation) whereas in
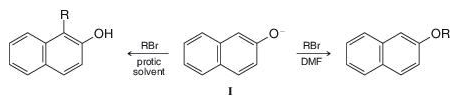
Why does the change in solvent make a difference?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you draw each step on a piece of a paper please this is very confusing to me
>
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
esc
?
A
O
O
•If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
• If your answer is no, check the box under the drawing area instead.
olo
18
Ar
Explanation
Check
BB
Click and drag to start drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
Name the structure
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - PRACTICE PROBLEM
21.1 If we examine Table 21.1, we...Ch. 21 - PRACTICE PROBLEM If we examine Table 21.1, we see...Ch. 21 - Prob. 3PPCh. 21 - PRACTICE PROBLEM
21.4 Predict the products of each...Ch. 21 - Prob. 5PPCh. 21 - PRACTICE PROBLEM
21.6 What are compounds A and B...Ch. 21 - Prob. 7PPCh. 21 - PRACTICE PROBLEM Outline a possible synthesis of...Ch. 21 - PRACTICE PROBLEM 1-Fluoro-2,4-dinitrobenzene is...Ch. 21 - PRACTICE PROBLEM
21.10 When o-chlorotoluene is...
Ch. 21 - PRACTICE PROBLEM When 2-bromo-1,3-dimethylbenzene...Ch. 21 - PRACTICE PROBLEM (a) Outline a step-by-step...Ch. 21 - Rank the following in order of increasing acidity.Ch. 21 - Prob. 14PCh. 21 - Prob. 15PCh. 21 - Describe a simple chemical test that could be used...Ch. 21 - Prob. 17PCh. 21 - Predict the product of the following reactions.Ch. 21 - 21.19 A synthesis of the β-receptor blocker called...Ch. 21 - Prob. 20PCh. 21 - When m-chlorotoluene is treated with sodium amide...Ch. 21 - Prob. 22PCh. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - Prob. 26PCh. 21 - Prob. 27PCh. 21 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Prob. 30PCh. 21 - Prob. 31PCh. 21 - 21.32 A compound X (C10H14O) dissolves in aqueous...Ch. 21 - 21.33 Compound Z (C5H10O) decolorizes bromine in...Ch. 21 - Explain why, in the case shown, the allyl group...Ch. 21 - In protic solvents the naphthoxide ion (I) is...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Prob. 40PCh. 21 - 21.41 Compound W was isolated from a marine...Ch. 21 - 21.42 Phenols generally are not changed on...Ch. 21 - 21.43 Open the molecular model file for benzyne...Ch. 21 - Which of the following would be the strongest...Ch. 21 - What products would you expect from the following...Ch. 21 - Prob. 3QCh. 21 - Prob. 4QCh. 21 - 21.5 Complete the following synthesis:
Ch. 21 - Prob. 6QCh. 21 - 21.7 Select the stronger acid.
Additional Science Textbook Solutions
Find more solutions based on key concepts
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
SYNTHESIZE YOUR KNOWLEDGE Watennelon snow in Antarctica is caused by a species of photosynthetic green algae th...
Campbell Biology (11th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
80 through 87 GO 80, 87 SSM WWW 83 Two-lens systems. In Fig. 34-45, stick figure O the object stands on the com...
Fundamentals of Physics Extended
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautome...
Organic Chemistry (8th Edition)
4. Two of these organ system bear the major responsibility for ensuring homeostasis of the internal environment...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forward
- Draw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forward
- Using the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forwardUsing arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forward
- Draw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forwardManoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forwardIn the table below, the correct structure for (2R)-3-methylpentan-2-ol (IUPAC name) can be represented by the letter OH OH HE > ' ÕH C B OH D A/ E OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY