
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 13P
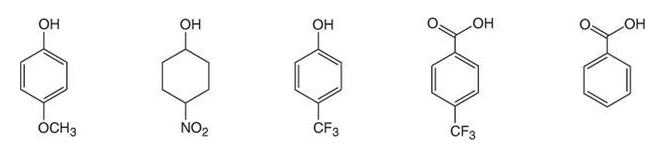
Rank the following in order of increasing acidity.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of this organic reaction.
If there aren't any products, because nothing will happen, check the box under the drawing area instead.
No reaction.
HO.
O
:☐
+
G
Na O.H
Click and drag to start
drawing a structure.
XS
xs H₂O
What are the angles a and b in the actual molecule of which this is a Lewis structure?
H
H C
H-
a
-H
b
H
Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal
groups may have slightly different sizes.
a =
b = 0 °
What are the angles a and b in the actual molecule of which this is a Lewis structure?
:0:
HCOH
a
Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that
different electron groups may have slightly different sizes.
a =
0
b=0°
S
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - PRACTICE PROBLEM
21.1 If we examine Table 21.1, we...Ch. 21 - PRACTICE PROBLEM If we examine Table 21.1, we see...Ch. 21 - Prob. 3PPCh. 21 - PRACTICE PROBLEM
21.4 Predict the products of each...Ch. 21 - Prob. 5PPCh. 21 - PRACTICE PROBLEM
21.6 What are compounds A and B...Ch. 21 - Prob. 7PPCh. 21 - PRACTICE PROBLEM Outline a possible synthesis of...Ch. 21 - PRACTICE PROBLEM 1-Fluoro-2,4-dinitrobenzene is...Ch. 21 - PRACTICE PROBLEM
21.10 When o-chlorotoluene is...
Ch. 21 - PRACTICE PROBLEM When 2-bromo-1,3-dimethylbenzene...Ch. 21 - PRACTICE PROBLEM (a) Outline a step-by-step...Ch. 21 - Rank the following in order of increasing acidity.Ch. 21 - Prob. 14PCh. 21 - Prob. 15PCh. 21 - Describe a simple chemical test that could be used...Ch. 21 - Prob. 17PCh. 21 - Predict the product of the following reactions.Ch. 21 - 21.19 A synthesis of the β-receptor blocker called...Ch. 21 - Prob. 20PCh. 21 - When m-chlorotoluene is treated with sodium amide...Ch. 21 - Prob. 22PCh. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - Prob. 26PCh. 21 - Prob. 27PCh. 21 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Prob. 30PCh. 21 - Prob. 31PCh. 21 - 21.32 A compound X (C10H14O) dissolves in aqueous...Ch. 21 - 21.33 Compound Z (C5H10O) decolorizes bromine in...Ch. 21 - Explain why, in the case shown, the allyl group...Ch. 21 - In protic solvents the naphthoxide ion (I) is...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Prob. 40PCh. 21 - 21.41 Compound W was isolated from a marine...Ch. 21 - 21.42 Phenols generally are not changed on...Ch. 21 - 21.43 Open the molecular model file for benzyne...Ch. 21 - Which of the following would be the strongest...Ch. 21 - What products would you expect from the following...Ch. 21 - Prob. 3QCh. 21 - Prob. 4QCh. 21 - 21.5 Complete the following synthesis:
Ch. 21 - Prob. 6QCh. 21 - 21.7 Select the stronger acid.
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. The event that triggered the change in...
Cosmic Perspective Fundamentals
Define rock deformation in your own words.
Applications and Investigations in Earth Science (9th Edition)
SCIENCE, TECHNOLOGY, AND SOCIETY In many countries, irrigation is depleting aquifers to such an extent that lan...
Campbell Biology (11th Edition)
103. What solution can you add to each cation mixture to precipitate one cation while keeping the other cation ...
Introductory Chemistry (6th Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the structures of the missing organic molecules in the following reaction: + H₂O +H OH O OH +H OH X Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactant in the following reaction: x + x O OH H* + ☑- X H+ O O Х Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H₂O) are not shown. In the drawing area below, draw the skeletal ("line") structure of the missing organic reactant X. Click and drag to start drawing a structure. Carrow_forwardCH3O OH OH O hemiacetal O acetal O neither O 0 O hemiacetal acetal neither OH hemiacetal O acetal O neither CH2 O-CH2-CH3 CH3-C-OH O hemiacetal O acetal CH3-CH2-CH2-0-c-O-CH2-CH2-CH3 O neither HO-CH2 ? 000 Ar Barrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 2 2. n-BuLi 3 Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure.arrow_forwardPredict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forwardPredict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forward
- Predict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H H+ Y Z ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. AP +arrow_forwardPlease help, this is all the calculations i got!!! I will rate!!!Approx mass of KMnO in vial: 3.464 4 Moss of beaker 3×~0. z Nax200: = 29.9219 Massof weacerv after remosimgain N2C2O4. Need to fill in all the missing blanks. ง ง Approx mass of KMnO4 in vials 3.464 Mass of beaker + 3x ~0-304: 29.9219 2~0.20 Miss of beaker + 2x- 29.7239 Mass of beaker + 1x~0.2g Naz (204 29-5249 Mass of beaver after removing as qa Na₂ C₂O T1 T2 T3 Final Buiet reading Initial butet reading (int)) Hass of NaOr used for Titration -reading (mL) calculation Results: 8.5ml 17mL 27.4mL Oml Om Oml T1 T2 T3 Moles of No CO Moles of KMO used LOF KM. O used Molenty of KMNO Averagem Of KMOWLarrow_forward
- Draw the skeletal ("line") structure of 2-hydroxy-4-methylpentanal. Click and drag to start drawing a structure. Xarrow_forwardDetermine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. hemiacetal acetal Oneither OHarrow_forwardWhat is the missing reactant R in this organic reaction? ་ ་ ་ ་ ་ ་ ་ ་ ་ ་ +R H3O+ • Draw the structure of R in the drawing area below. N • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY