
Concept explainers
PRACTICE PROBLEM
If we examine Table 21.1, we find that the methylphenols (cresols) are less acidic than phenol itself. For example,
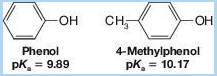
This behavior is characteristic of phenols bearing electron-releasing groups. Provide an explanation.
Interpretation:
Methyl phenols are less acidic than phenols, due to the presence of an electron releasing group on them. The statement is to be explained.
Concept introduction:
Phenols are compounds of benzene, bearing a hydroxyl group. They are alcohol derivatives but have higher acidities than alkyl alcohols.
Methyl phenols are phenol derivatives, in which a hydrogen atom attached to the carbon ring is replaced by a methyl
Answer to Problem 1PP
Solution: The
Explanation of Solution
According to table
Phenol is more acidic, as the
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
Campbell Biology (11th Edition)
Campbell Essential Biology with Physiology (5th Edition)
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
