
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 5Q
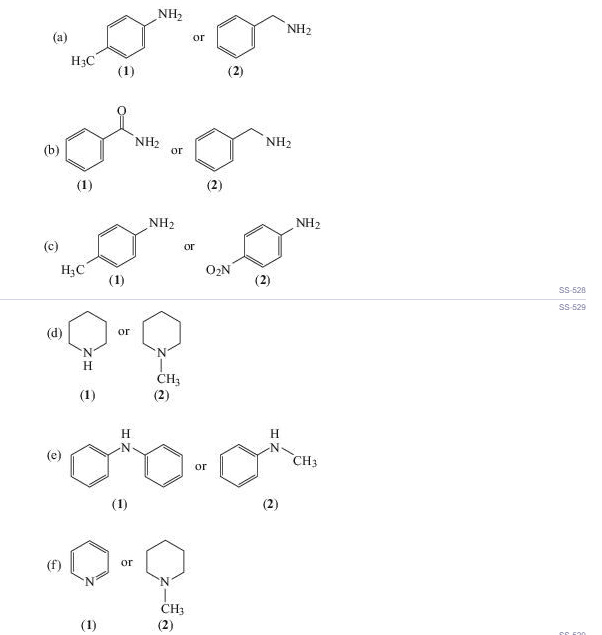
Select the stronger base from each pair (in aqueous solution):
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 20 Solutions
Organic Chemistry
Ch. 20 - Prob. 1PPCh. 20 - Prob. 2PPCh. 20 - Prob. 3PPCh. 20 - PRACTICE PROBLEM 20.5 Outline a preparation of...Ch. 20 - Prob. 5PPCh. 20 - Prob. 6PPCh. 20 - Prob. 7PPCh. 20 - Prob. 8PPCh. 20 - Prob. 9PPCh. 20 - Practice Problem 20.11 In the preceding examples...
Ch. 20 - Prob. 11PPCh. 20 - Prob. 12PPCh. 20 - Practice Problem 20.14
Outline a synthesis of...Ch. 20 - Prob. 14PPCh. 20 - Prob. 15PPCh. 20 - PRACTICE PROBLEM
20.16
An amine A has the...Ch. 20 - PRACTICE PROBLEM Sulfonamides of primary amines...Ch. 20 - PRACTICE PROBLEM
20.18 (a) Starting with aniline...Ch. 20 - Prob. 19PCh. 20 - 20.20 Give common or systematic names for each of...Ch. 20 - Which is the most basic nitrogen in each compound?...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Show how you might synthesize each of the...Ch. 20 - Prob. 25PCh. 20 - 20.26 Provide the major organic product from each...Ch. 20 - Prob. 27PCh. 20 - Prob. 28PCh. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Write equations for simple chemical rests or state...Ch. 20 - Prob. 33PCh. 20 - 20.34 Using reactions that we have studied in this...Ch. 20 - 20.35 Provide a detailed mechanism for each of the...Ch. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - 20.40 Give structures for compounds R-W:
Ch. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Diethylpropion (shown here) is a compound used in...Ch. 20 - Prob. 44PCh. 20 - 20.45 Compound W is soluble in dilute aqueous HCI...Ch. 20 - 20.46 Propose structures for compounds X, Y, and...Ch. 20 - Compound A(C10H15N) is soluble in dilute HCI. The...Ch. 20 - Prob. 48PCh. 20 - Prob. 49PCh. 20 - 20.52 When phenyl isochiocyanatc, , is reduced...Ch. 20 - Prob. 51PCh. 20 - 20.54 Propose a mechanism that can explain the...Ch. 20 - When acetone is treated with anhydrous ammonia in...Ch. 20 - Prob. 54PCh. 20 - Which of the following would be soluble in dilute...Ch. 20 - Which would yield propylamine? (d) Two of the...Ch. 20 - Select the reagent from the list below that could...Ch. 20 - Prob. 4QCh. 20 - 20.5 Select the stronger base from each pair (in...
Additional Science Textbook Solutions
Find more solutions based on key concepts
A mixed culture of Escherichia coli and Penicillium chrysogenum is inoculated onto the following culture media....
Microbiology: An Introduction
41. A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero ord...
Chemistry: Structure and Properties (2nd Edition)
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
10.1 Indicate whether each of the following statements is characteristic of an acid, a base, or
both:
has a so...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
SCIENCE, TECHNOLOGY, AND SOCIETY In many countries, irrigation is depleting aquifers to such an extent that lan...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY