
a)
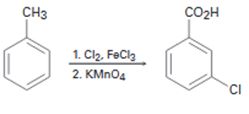
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, alkyl groups are ortho and para directing and activating groups while
To state:
The flaw in the synthesis given.
b)
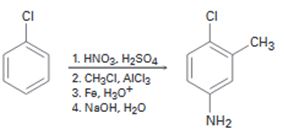
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, halogens are ortho and para directing groups. Friedal-Crafts alkylation is not possible with
To state:
The flaw in the synthesis given.
c)
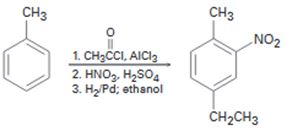
Interpretation:
The flaw in the synthesis given is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, alkyl groups are ortho and para directing and activating groups while carbonyl group is a meta directing and deactivating group. When treated with H2/Pd the –C=O group gets reduced to –CH2 group and –NO2 gets reduced to –NH2 group.
To state:
The flaw in the synthesis shown.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- (2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward8. (2 pts) Silicon semiconductors have a bandgap of 1.11 eV. What is the longest photon wavelength that can promote an electron from the valence band to the conduction band in a silicon-based photovoltaic solar cell? Show all work. E = hv = hc/λ h = 6.626 x 10-34 Js c = 3.00 x 108 m/s 1 eV 1.602 x 10-19 Jarrow_forward
- A solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell: calomel electrode (saturated)//titration solution/Hg(l) Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E. Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V E' (calomel electrode, saturated KCl) = 0.241 Varrow_forwardFrom the following reduction potentials I2 (s) + 2e- = 2I- (aq) E0= 0.535 V I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq). b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq). c) Calculate the solubility of I2 (s) in water.arrow_forward2. (3 pts) Consider the unit cell for the spinel compound, CrFe204. How many total particles are in the unit cell? Also, show how the number of particles and their positions are consistent with the CrFe204 stoichiometry - this may or may not be reflected by the particle colors in the diagram. (HINT: In the diagram, the blue particle is in an interior position while each red particle is either in a corner or face position.)arrow_forward
- From the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forwardCalculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forwardA solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward
- 1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O2/HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI + enant OH Solvent Reagent(s) Solvent Reagent(s)arrow_forwardGermanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

