
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.SE, Problem 74AP
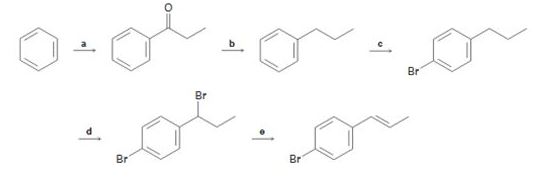
Identify the reagents represented by the letters a-e in the following scheme:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Given the hydrazones indicated, draw the structures of the enamines that
can be formed. Indicate the most stable enamine (explain).
C6H5
C6H5
H
C6H5
H
4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6
carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not
count towards this total, and the starting material can have whatever non-carbon functional
groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and
III. Your final answer should show each step separately, with intermediates and conditions clearly
drawn.
Indicate the importance of the indole ring. Find a representative example and list 5 structures.
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - Prob. 1PCh. 16.2 - Propose a mechanism for the electrophilic...Ch. 16.2 - How many products might be formed on chlorination...Ch. 16.2 - When benzene is treated with D2SŪ4. deuterium...Ch. 16.3 - Prob. 5PCh. 16.3 - What is the major monosubstitution product from...Ch. 16.3 - Identify the carboxylic acid chloride that might...Ch. 16.4 - Rank the compounds in each of the following groups...Ch. 16.4 - Predict the major products of the following...Ch. 16.4 - Prob. 10P
Ch. 16.4 - Prob. 11PCh. 16.4 - Acetanilide is less reactive than aniline toward...Ch. 16.4 - Prob. 13PCh. 16.5 - At what position would you expect electrophilic...Ch. 16.5 - Show the major product(s) from reaction of the...Ch. 16.6 - The herbicide oxyfluorfen can be prepared by...Ch. 16.7 - Treatment of p-bromotoluene with NaOH at 300°C...Ch. 16.8 - Prob. 18PCh. 16.8 - Prob. 19PCh. 16.8 - Prob. 20PCh. 16.9 - Prob. 21PCh. 16.10 - Prob. 22PCh. 16.10 - Prob. 23PCh. 16.SE - Prob. 24VCCh. 16.SE - The following molecular model of a...Ch. 16.SE - Prob. 26VCCh. 16.SE - Prob. 27VCCh. 16.SE - Aromatic iodination can be carried out with a...Ch. 16.SE - Prob. 29MPCh. 16.SE - The carbocation electrophile in a Friede1-Crafts...Ch. 16.SE - Prob. 31MPCh. 16.SE - The nitroso group, —N=O, is one of the few...Ch. 16.SE - Triphenylmethane can be prepared by reaction of...Ch. 16.SE - Using resonance structures of the intermediates,...Ch. 16.SE - Benzene and alkyl -substituted benzenes can be...Ch. 16.SE - Prob. 36MPCh. 16.SE - Hexachlorophene, a substance used in the...Ch. 16.SE - Benzenediazonium carboxylate decomposes when...Ch. 16.SE - 4-Chloropyridine undergoes reaction with...Ch. 16.SE - Propose a mechanism to account for the following...Ch. 16.SE - In the Gatterman-Kochreaction, a formyl group...Ch. 16.SE - Treatment of p-tert-butylphenol with a strong acid...Ch. 16.SE - Benzyl bromide is converted into benzaldehyde by...Ch. 16.SE - Prob. 44MPCh. 16.SE - Prob. 45MPCh. 16.SE - Prob. 46APCh. 16.SE - Prob. 47APCh. 16.SE - Prob. 48APCh. 16.SE - Predict the major monoalkylation products you...Ch. 16.SE - Name and draw the major product(s) of...Ch. 16.SE - Prob. 51APCh. 16.SE - Prob. 52APCh. 16.SE - What product(s) would you expect to obtain from...Ch. 16.SE - Prob. 54APCh. 16.SE - How would you synthesize the following substances...Ch. 16.SE - Prob. 56APCh. 16.SE - Prob. 57APCh. 16.SE - Prob. 58APCh. 16.SE - Prob. 59APCh. 16.SE - Prob. 60APCh. 16.SE - Prob. 61APCh. 16.SE - Prob. 62APCh. 16.SE - Prob. 63APCh. 16.SE - How would you synthesize the following substances...Ch. 16.SE - Prob. 65APCh. 16.SE - Prob. 66APCh. 16.SE - Draw resonance structures of the intermediate...Ch. 16.SE - Prob. 68APCh. 16.SE - p-Bromotoluene reacts with potassium amide to give...Ch. 16.SE - Prob. 70APCh. 16.SE - Prob. 71APCh. 16.SE - Prob. 72APCh. 16.SE - Use your knowledge of directing effects, along...Ch. 16.SE - Identify the reagents represented by the letters...Ch. 16.SE - Phenols (ArOH) are relatively acidic, and the...Ch. 16.SE - Prob. 76APCh. 16.SE - Prob. 77APCh. 16.SE - Melamine, used as a fire retardant and a component...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ΌΗ 1) V2 CO 3 or Nalt In منهarrow_forward6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forward
- Consider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forwardGiven Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forward
- Hydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward(3 pts) Use the Kapustinskii equation to calculate the lattice enthalpy for MgBr2 anddiscuss any differences between this result and that from #4.arrow_forward(3 pts) Silver metal adopts a fcc unit cell structure and has an atomic radius of 144 pm. Fromthis information, calculate the density of silver. Show all work.arrow_forward
- 4. (3 pts) From the information below, determine the lattice enthalpy for MgBr2. Show all work. AH/(kJ mol-¹) Sublimation of Mg(s) +148 lonization of Mg(g) +2187 to Mg2+(g) Vaporization of Br₂(1) +31 Dissociation of Br,(g) +193 Electron gain by Br(g) -331 Formation of MgBr₂(s) -524arrow_forward1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License