
Concept explainers
(a)
Interpretation:
The product formed from the following
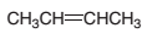
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is called a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
(b)
Interpretation:
The product formed from the following alkene on reacting with
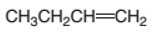
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is called a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
(c)
Interpretation:
The product formed from the following alkene on reacting with
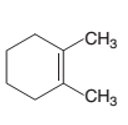
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is called a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General, Organic, & Biological Chemistry
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



