
Concept explainers
(a)
Interpretation:
The IUPAC name for the following alkene should be determined:
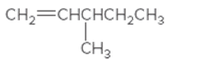
Concept Introduction:
(b)
Interpretation:
The IUPAC name for the following alkene should be determined:
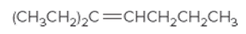
Concept Introduction:
Alkenes are named in the same way as alkanes, but the alkenes are identified by the suffix −ene, replaced instead of the ending of the name of the parent alkane. The longest carbon chain should be numbered in a way that gives the double bond the lower number. Then the compound should be named using the first number assigned to the double bond. The names of the substituents should be written first in the alphabetic order with their position on the chain.
(c)
Interpretation:
The IUPAC name for the following alkene should be determined:
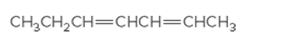
Concept Introduction:
Alkenes are named in the same way as alkanes, but the alkenes are identified by the suffix −ene, replaced instead of the ending of the name of the parent alkane. The longest carbon chain should be numbered in a way that gives the double bond the lower number. Then the compound should be named using the first number assigned to the double bond. The names of the substituents should be written first in the alphabetic order with their position on the chain.
(d)
Interpretation:
The IUPAC name for the following alkene should be determined:
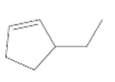
Concept Introduction:
In the nomenclature of cycloalkenes, the double bond is always located between C-1 and C-2. And 1 is not mentioned in the name. the ring is then numbered in a way that the first substituent getting the lowest number.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General, Organic, & Biological Chemistry
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

